Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Postulates of Kinetic Particle Theory of gases
- A relationship between temperature & volume of a gas
- A relationship between external pressure & volume of a gas
Kinetic Particle Theory of Gases: Postulates
This theory has the following postulates related to gases.
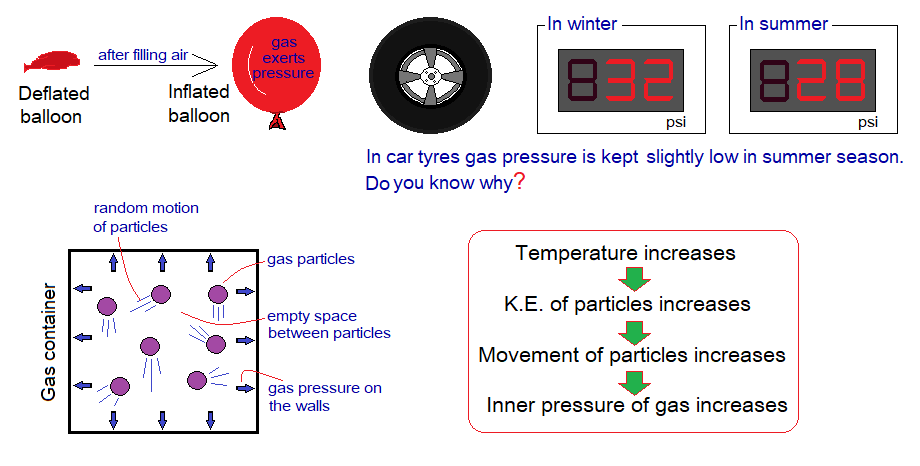
- Gases are made up of tiny particles which are far apart from each other, have a lot of space between.
- These particles move with constant and random motion.
- These particles create an inside pressure of the gas which is imposed on the walls of the container.
- By increasing the temperature, the gas pressure increases. The kinetic energy of particles is increased by rising up the temperature; resultantly, the particles’ motion increases.
- The volume of the gas is increased by increase in temperature; and vice versa.
- The volume of the gas is decreased by increase in external pressure; and vice versa.
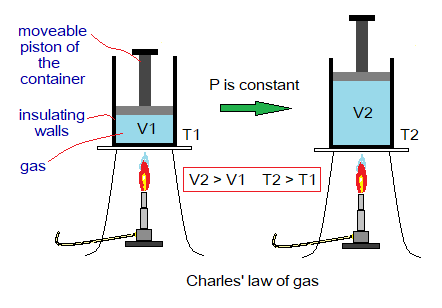
Temperature & Volume of Gas: Relationship
By a simple definition, “the volume of a gas has a direct relationship with temperature at constant pressure”. Mathematically, it would be expressed as:

The ‘α – alpha’ is a Greek alphabet that is used as a symbol to show relationship between two variables (here, V & T).
This relationship was formulated by Jacques Alexandre Cesar Charles (1746-1823) in 1787. So, this law is known as Charles law of gases. He was French professor of science and flew a world first balloon in 1783 by working with two brothers Anne-Jean Robert and Les Freres Robert, who were engineers.
Suppose, V1 is initial volume of a gas at temperature T1. When temperature of the gas is increased by T2, the volume of the gas is also increased by V2 at constant external pressure. In other words, the gases expand by rising the temperature; aka thermal expansion of gases.
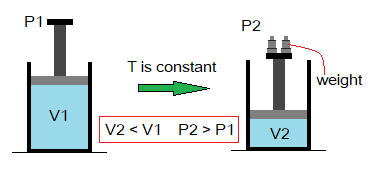
External Pressure & Volume of Gas: Relationship
By a simple definition, “the volume of a gas has an indirect relationship with applied (external) pressure at constant temperature”. Mathematically, it would be expressed as:
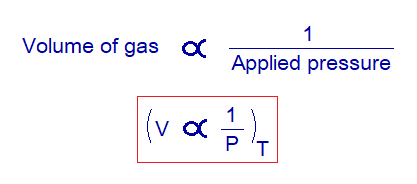
This relationship was formulated by an Irish scientist and philosopher Robert Boyle (1627-1691) in 1662. So, this law is known as Boyle’s law of gases.
Suppose, V1 is initial volume of a gas at pressure P1. When external pressure on the gas is increased by P2, the volume of the gas is decreased by V2 at constant temperature. In the diagram below, it is shown that the weights are used on the moveable piston to apply pressure on the gas. By the conclusion, it is verified that the volume of the gases has inverse relationship with applied pressure.