Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Phase change
- Heating curve
- Cooling curve
- Other examples
Phase Changes:
This article describes heating and cooling curves based upon water at 1 atmospheric pressure (sea level). The link https:/change-of-state-of-matter/ describes what is phase and its change. Further, melting point, freezing point, boiling point and condensation points have been explained on this link. When a substance changes its appearance (phase) it needs or releases energy in the form of heat. By this, there are two types of curves formed, heating and cooling curves.
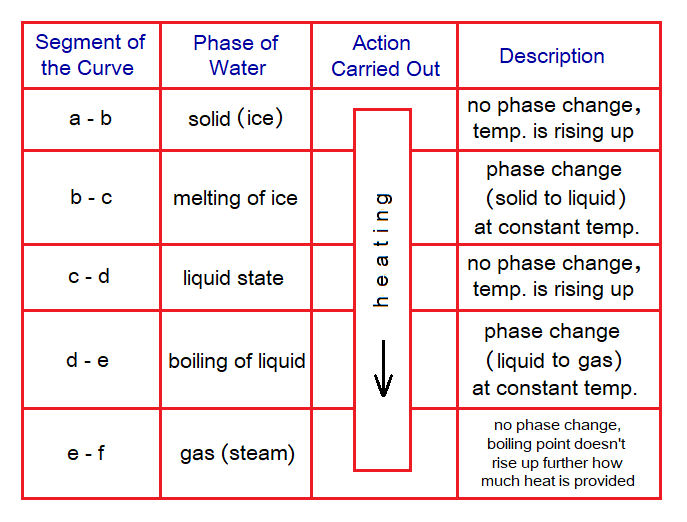
Heating Curve:
Following table explains the segments of the curve for water starting from ice to its gaseous phase.
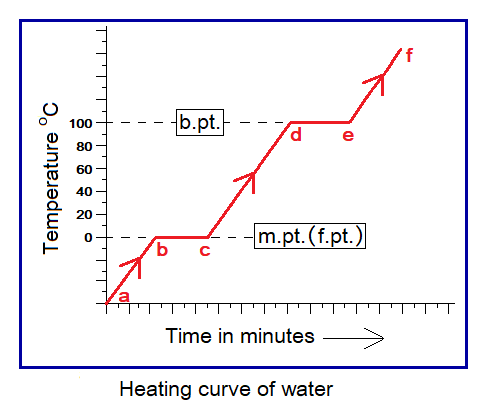
For ice, at point b the 0°C is the temperature where phase change begins to come about as shown in the heating curve. The b to c is a flat segment and temperature remains constant. The phase change takes place during this flat segment which takes time for ice to be melted. From point c, at 0°C, the temperature of the liquid state again starts going up towards 100°C. At point d, 100°C is the temperature where the curve again shows a flat segment and the temperature remains constant but time goes on and at point e liquid starts boiling and converting to gas.
Cooling Curve:
Following table explains the segments of the curve for water starting from steam to the formation of ice.
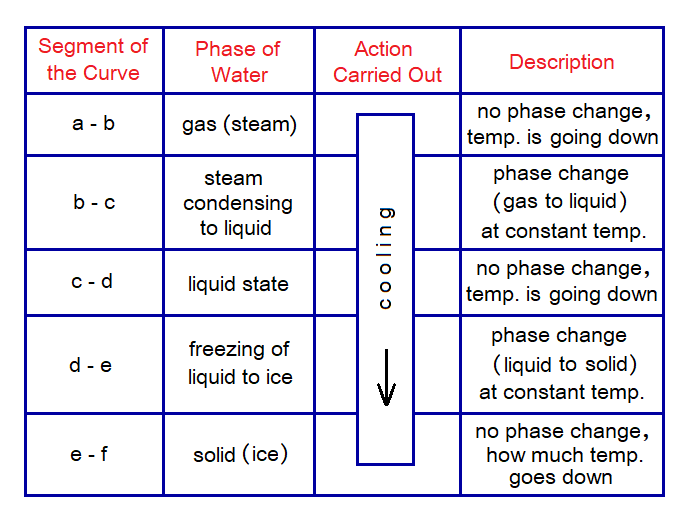
For steam, at point b the 100°C is the temperature where phase change begins to come about as shown in the cooling curve. The b to c is a flat segment and temperature remains constant. The phase change takes place during this flat segment which takes time and the steam condenses to liquid. From point c, at 100°C, the temperature of the liquid state again starts going down towards 0°C. At point d, 0°C is the temperature where the curve again shows a flat segment and the temperature remains constant but time goes on and at point e liquid starts converting to ice.
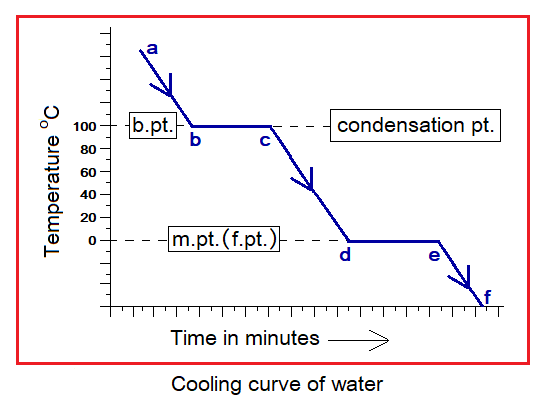
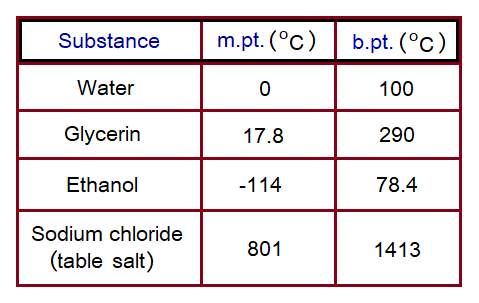
Other Examples:
Various substances have their own melting and boiling points. Few examples are given here at 1 atmospheric pressure in the following table. Their heating and cooling curves are formed depending upon these values.