Dr. Mudassar Altaf, Associate Professor, Higher Education Department, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Evaporation – definition
- Examples of evaporation
- Characteristics of evaporation
- Evaporation & condensation in climate
Evaporation – Definition:
The etymology of this term is Latin ‘evaporare’ meaning ‘to disappear in the form of vapours’. So, this term is defined as, “the phenomenon by which liquid disappears in the form of vapours below its boiling point”.
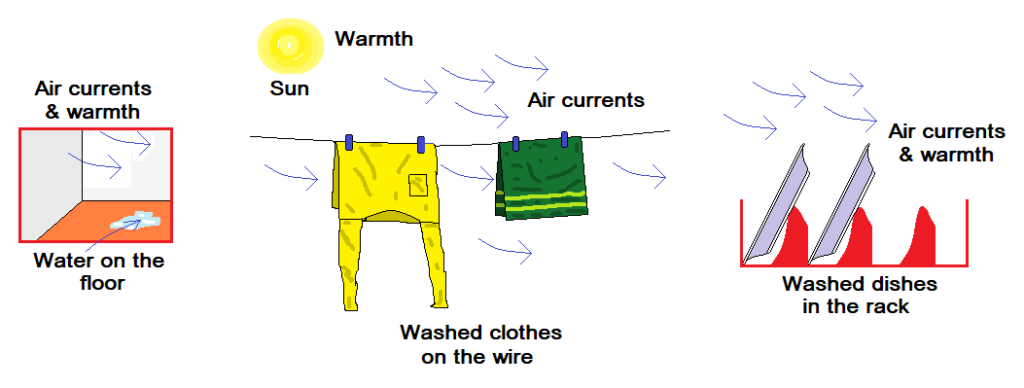
Examples:
- It is a common observation that washed clothes when hanged on wire get dried.
- Washed dishes in kitchen dry after some time.
- The water present on the floor disappears.
Characteristics of Evaporation:
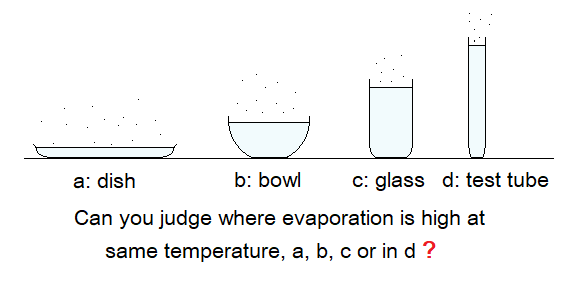
- Evaporation is a surface phenomenon. More the surface of the liquid, more would be the evaporation.
- Evaporation takes place at all temperature. However, its rate increases on rise of temperature.
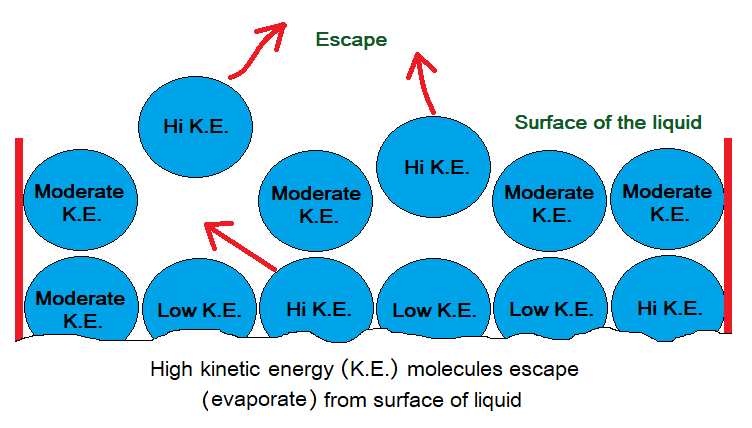
- High kinetic energy molecules escape from the surface. The molecules that evaporate certainly absorb energy, thus attain high kinetic energy.
- Evaporation is a phenomenon takes place below the boiling point of the liquid.
- Evaporation is a natural phenomenon and its existence is a necessary part of the climate.
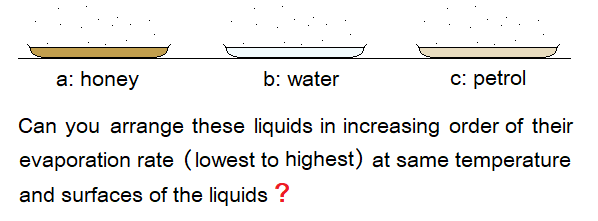
- Different liquids have different rate of evaporation. Thick liquids have very low rate as compared to more volatile ones. A honey is thicker than water. Water is thicker than petrol. Volatile are those liquids that can easily be poured into another container. Volatile by etymology has its origin from Latin ‘volatilis’ meaning’ flying creature’. So, volatile is a term coined for such liquids having good evaporation rate.
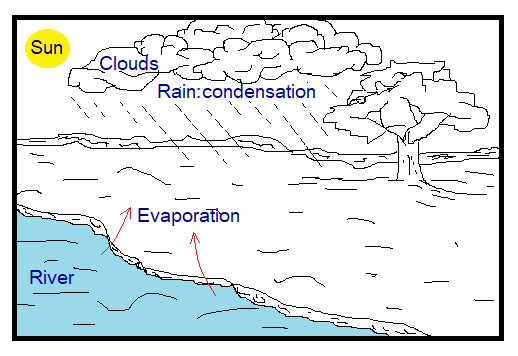
Evaporation & Condensation in Climate:
Water molecules escape from the surface of the water bodies of the earth due to heat by the sun. The water vapours form humidity in the atmosphere. They go up and form clouds. By condensation at the altitude the raindrops fall on the earth surface. So, this is a cyclic process of the nature. For the study of condensation can go onto the link https:/change-of-state-of-matter/