Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Diffusion: introduction and definition
- Kinetic Particle Theory and diffusion
Diffusion: Introduction & Definition:
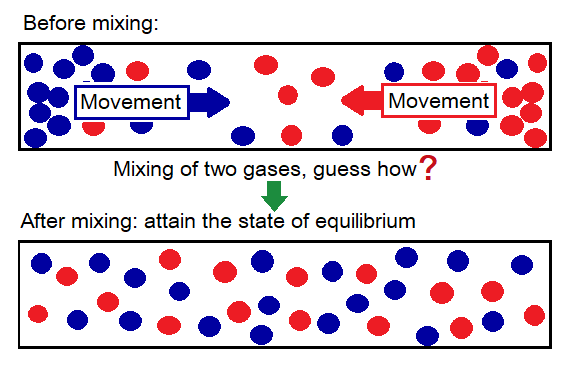
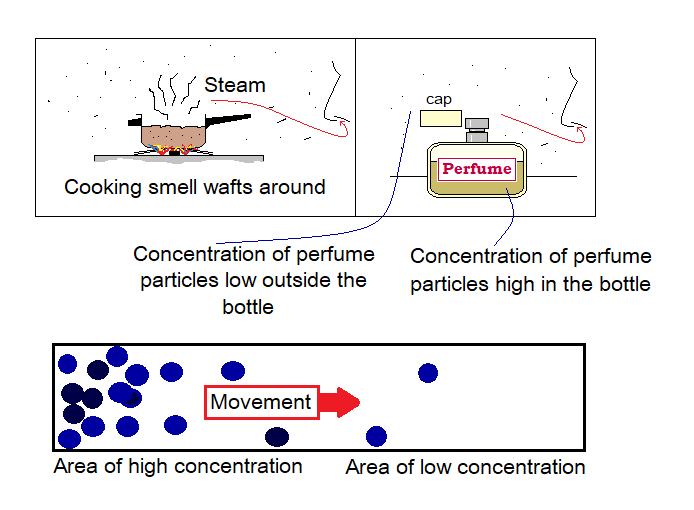
The Latin word ‘diffundere’ is a root of the term ‘diffusion. The meaning of the diffundere is ‘to spread out, to disperse, to scatter’. We can smell the perfume from the distance because, its particles permeate in the air. By definition the diffusion can be defined as, “the spreading of the particles of matter towards the area of lower concentration from the area where their concentration is higher by means of kinetic energy and the random motion”. Concentration means where the ‘gathering of particles’ of a particular matter exists either low or high.
Kinetic Particle Theory & Diffusion:
Diffusion takes place in solids, liquids, and gases. Rate (speed to move) of diffusion is high in gases, slow in solids while moderate in liquids. Volatile liquids show high rate of diffusion as compared to non-volatile ones. To have a brief introduction of volatile liquids click https:/evaporation-condensation/
According to KPT:
- Particles of the gases move with constant and random motion. This movement is slow in liquids and in solids they just vibrate at their fix positions.
- Particles need kinetic energy for their movement. Higher the kinetic energy, higher will be the speed to move.
- Kinetic energy is directly related with temperature. Higher the temperature, higher will be the kinetic energy of the particles.
- The collision between particles is elastic, means there is no gain or loss of energies by collision.
- Particles of different substances mix with one another by means of diffusion and make a mixture. By diffusion they move from higher to lower of their concentration.