Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Reversible reactions (bi-directional): definition
- Changing the condition can change direction
- Reversible reaction is at equilibrium
- Dynamic equilibrium
- Le-Chatelier’s principle: definition
- Effect of temperature change on reversible reactions
- Effect of pressure change on reversible reactions
- Effect of concentration change on reversible reactions
- Effect of catalyst on reversible reactions
- Synthesis of ammonia by Haber process
- Le-Chatelier’s principle in ammonia synthesis
- Safety considerations in Haber process
- Economic considerations of Haber process
- Synthesis of sulfuric acid by contact process
- Le-Chatelier’s principle in SO3 synthesis
- Safety considerations in contact process
- Economic considerations of contact process
Reversible Reactions:
All reactions are not reversible. The reactions that are reversible can be stated as: Definition: “The chemical reactions that can go forward as well as backward under the same conditions” are called reversible reactions. The reactants form products, is forward going direction; while the products start forming on reverse the reactants, is backward going direction. Such type of chemical equation is expressed by having double arrow in-between reactants and products.

Changing the Condition Can Change Direction:
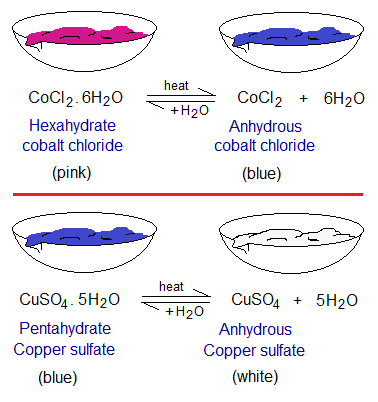
Reversible reactions are bi-directional. This direction is conditional; and the change decides to which direction the reaction will go. Consider the hydrated and anhydrous compounds.
- Anhydrous (water-free) cobalt (II) chloride is a blue crystalline powder in its appearance. The hydrated form of this salt is pink. When blue absorbs moisture, it turns pink; so, used as moisture indicator. However, when it is heated, it turns again to blue due to reverse reaction by removing water of crystallization. The water as a part of crystalline structure in hydrated ionic compounds is called water of crystallization; as in CoCl2.6H2O, these 6 water molecules; and these are written after molecular formula having a dot in-between. It is hexahydrate, means 6 water molecules per formula unit. This compound is carcinogenic (cancer forming) and unsuitable for use.
- Anhydrous copper (II) sulfate is a white crystalline powder in its appearance. The hydrated form of this salt is blue. When white absorbs water, turns blue. However, when it is heated, it dehydrates again to white powder. It is pentahydrate, means 5 water molecules per formula unit. It is used as desiccant (a drying agent). Desiccant has its Latin origin from the word ‘desiccare’ meaning ‘making dry’.
Reversible Reaction is at Equilibrium:
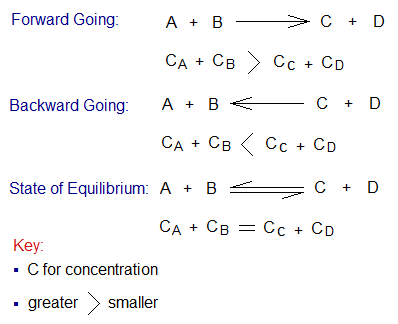
- In a closed container, the reversible reactions are at equilibrium between reactants and products. In other words, rate of forward reaction is equal to the rate of backward reaction.
- When the reactants are in greater concentration, the reaction goes forward and forms the products.
- When the products become higher in concentration, the reaction starts going backward (reverse) and forming reactants again.
- At the midway between 2 & 3 above, there comes an equilibrium state; i.e., rate of forward reaction becomes equal to the rate of backward reaction. The reaction attains a state of dynamic equilibrium.
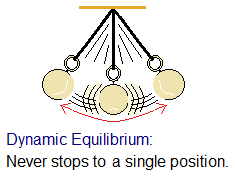
Dynamic Equilibrium: It is stated that, “when rates of forward and backward directions are equal and not under static condition” is said to be dynamic equilibrium. In other words, the reaction doesn’t stop at any moment but moves in either direction like a pendulum-swing and imagine if it doesn’t stop. The ‘dynamis’ is a Greek word having the meanings of ‘strength, power’. So, in terms of equilibrium, it might be ‘the chemical reaction having a strength to move non-stop bi-directional’. To the speed (rate) the reaction going forward, to the same speed the reaction has its reverse direction; and this state specifies the dynamic equilibrium.
Le-Chatelier’s Principle:
Definition: This principle is defined as, “when a change is applied on a system at equilibrium, the system adjusts itself to minimize the effect of the applied change in order to reestablish equilibrium state”. This applied effect might be due to change in concentration, increase or decrease in temperature or pressure, as described below in detail. Henry Louis Le-Chatelier (1850-1936) was a French chemist who proposed this principle in 1884.
Effect of Temperature Change on Reversible Reactions:
Chemical reactions are either exothermic or endothermic. If forward going direction is exothermic then reverse will be endothermic and vice versa; having the same ∆H (change in enthalpy) but with opposite signs. In general, rising temperature favours endothermic; while lowering favours exothermic.
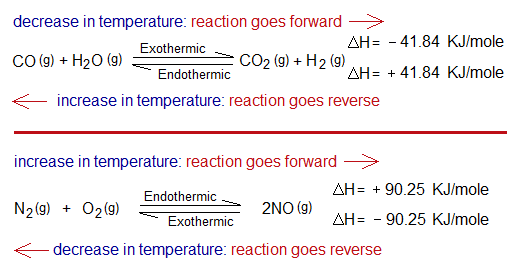
- Consider the synthesis of CO2 from CO and steam. It is exothermic in forward going direction; so, would be endothermic when reforms the initial reactants. Decrease in temperature will shift the reaction towards right side; however, increase in temperature will shift the reaction in opposite direction. Look at ∆H, -41.84 KJ/mole for exothermic; and the same value but positive for endothermic.
- Consider another system, the synthesis of NO gas (nitric oxide, nitrogen monoxide) by chemical reaction between N2 & O2 gases. It is endothermic in forward direction; while exothermic in reverse. So, the decrease in temperature will shift the reaction towards left side; and increase in temperature will shift the reaction in opposite side; having the same ∆H, but with opposite signs.
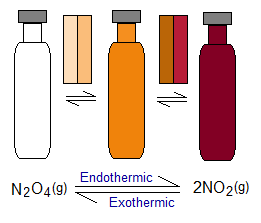
- Dinitrogen tetraoxide (N2O4) is a colourless gas; while nitrogen dioxide (NO2) gas is dark brown. N2O4 conversion to NO2 is endothermic; while reverse is exothermic. So, at higher temperature (50°C) the brown appearance of the glass vial will show the higher concentration of NO2; while at very lower temperature, this dark colour fades and even at -196°C, the colourless appearance of the vial will show the presence of N2O4.
Effect of Pressure Change on Reversible Reactions:
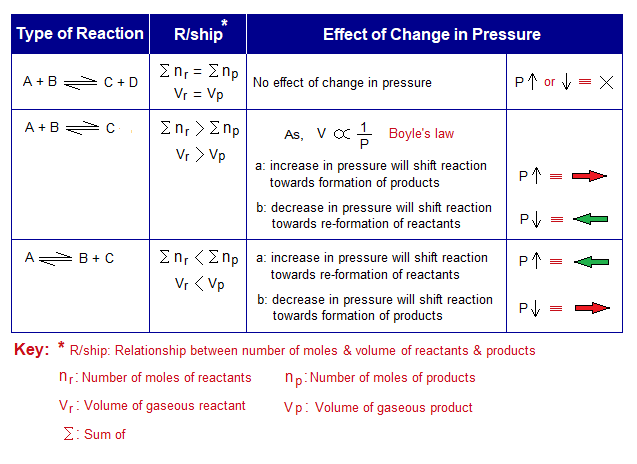
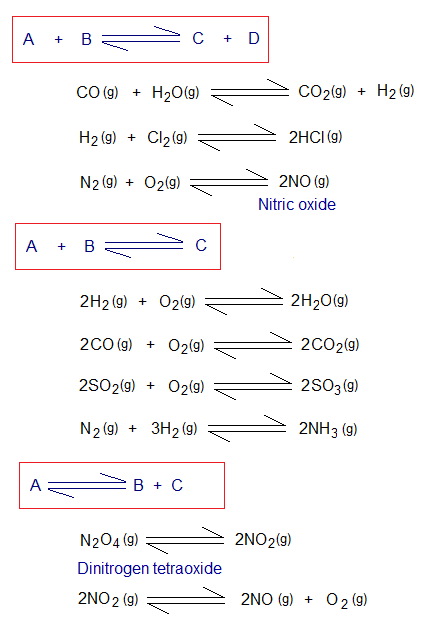
The effect of pressure is studied on gaseous systems with its relationship with the change in volume. This relationship is a narrative of Boyle’s law which serves as a prototype and states that “the volume of an ideal gas is inversely related with the applied pressure on the system when the temperature is kept constant”. For In-detail study go to the link: https://chemiologist.com/effects-of-temperature-pressure-on-gas-volume-an-explanation-by-kpt/ . In a cylinder that is equipped with a movable piston, the volume of the gas is decreased by increasing the external pressure, and vice versa. Similarly, this relationship is applied on the reversible chemical equations when reactants and products are gases. The number of moles decide where the volume is higher or lower or might be equal. Upon this criterion, the chemical equations can be classified into three categories, as given under:
- If number of moles between reactants and products are equal, then there will be no effect of applied pressure on the system.
- If number of moles of reactants are greater then, (a) the increase in pressure will shift the reaction forward (towards right side), i.e., will be in favour of formation of products; because of keeping the lower volume on right side. (b) the decrease in pressure will shift the reaction backward direction (reverse, towards left side), i.e., will be in favour of reformation of initial reactants; because of keeping the higher volume on the left side.
- If number of moles of products are greater then, (a) the increase in pressure will shift the reaction backward direction, i.e., will be in favour of formation of reactants; because of keeping the lower volume on that side. (b) the decrease in pressure will shift the reaction forward, i.e., will be in favour of synthesis of products; because of keeping the higher volume on that side.
Below are a few common examples:
Effect of the Concentration Change on Reversible Reactions:
The concentrations of reactants and products in the reaction flask also determine the direction of the reversible reaction at a particular time; because, the change is always dynamic. If the concentration of reactants is greater, then, the direction will be forward, and vice versa to minimize the effect of the higher concentration at that side and to establish equilibrium state again according to Le-Chatelier’s principle. Following are two examples given in this context.
Example 1:
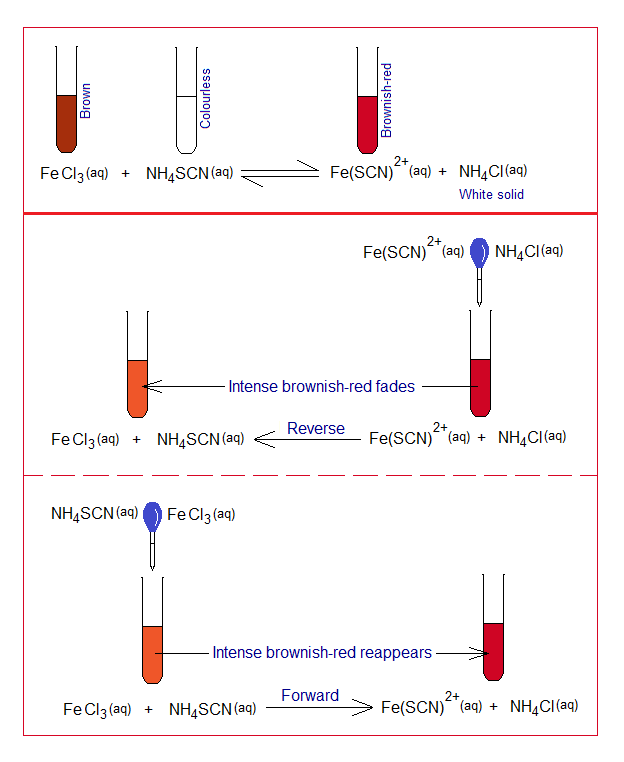
- The aqueous solution of ferric chloride (FeCl3) is brown; however, the solution of ammonium thiocyanate (NH4SCN) in water is colourless.
- Adding drop-by-drop the ferric chloride solution into ammonium thiocyanate colourless solution, the intense (deep) brownish-red colour appears due to formation of a complex molecule of iron (III) thiocyanate [Fe(SCN)2+]; and the other product is ammonium chloride. This is forward going direction.
- Because, the reaction is reversible, so, to check the effect of the concentrations, add few drops of aqueous ammonium chloride solution into the test-tube containing the intense brownish-red colouration. This tone will fade due to reverse direction of the chemical reaction.
- Following that, add few drops of initial reactants into the test-tube containing fade colouration, the tone will again turn to intense brownish-red due to forward direction by this.
Example 2:
Phenolphthalein is a white powder organic compound. Its solution in ethanol is used in acid-base titration as an indicator.
- Take 5 ml of NaOH (0.1 molar) solution in a beaker; and add 1 drop of phenolphthalein indicator. The pink solution will be appeared; because, phenolphthalein gives pink colour in basic media.
- Now, drop-by-drop add HCl solution (0.1 molar) into the pink solution. The pink shade of the solution will start fading and ultimately will become colourless. Because, the phenolphthalein is colourless in acidic media. As, the acid is more in quantity than base.
- Now, to the above colourless solution add drop-by-drop the base solution; when, its quantity will exceed to the quantity of the acid in the beaker, then, the phenolphthalein will again turn to pink.
There is a reversible structural rearrangement in phenolphthalein molecule in acidic and basic media. It hasn’t been discussed here.

Effect of Catalyst on Reversible Reactions:
There is no effect of catalyst on the position of equilibrium of reversible reactions. The main concern of catalyst is to increase the rate (speed) for its occurrence by lowering the energy of activation.
Synthesis of Ammonia by Haber Process:
Fritz J. Haber (1868-1934) and Carl Bosch (1874-1940), both were German chemists and Nobel Laureate in chemistry, developed an industrial process for the synthesis of ammonia gas in 1909. So, the process is simply called Haber Process but, it is Haber-Bosch Process by its another name. BASF- a German chemical manufacturing company purchased the method and started their manufacturing. The simple step-by-step procedure is as under:
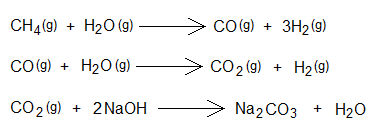
- Synthesis of hydrogen gas from methane: Hydrogen gas is obtained by the chemical reaction between methane and steam at high temperature and at high pressure in the presence of catalyst. The chemical reaction produces carbon-monoxide and hydrogen gases, a mixture called water gas. CO gas further reacts with steam to produce gases, CO2 and more H2. CO2 gas is removed by its conversion to sodium carbonate (Na2CO3) by reaction with NaOH.
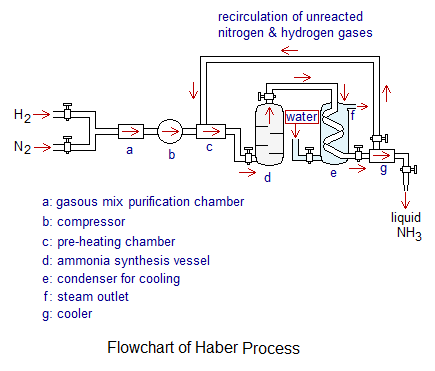
- Nitrogen gas is Obtained from Air: The air is liquified by compression and cooling; and the clean and dry liquid air is then warmed slowly in a fractionating column to separate nitrogen at its boiling point of -196°C. It is obtained 99.9% pure.
- Synthesis of Ammonia: The compressed gaseous mixture of N2 & H2 is pre-heated and introduced into Ammonia Synthesis Vessel. It is a strong thick-walled steel vessel, also called Haber convertor or reactor. Inside the vessel, there are several beds of catalysts; where finally divided iron as a catalyst is placed along with catalysts’ promotors potassium oxide (K2O) and aluminum oxide (Al2O3). The gases pass through these beds and chemical conversion takes place at 450-500°C temperature under pressure of 200 atmosphere (20265 kilo-Pascal: kpa). As, 1 atm pressure = 101.325 kpa. The reaction between N2 & H2 is exothermic with the liberation of heat ∆H= -92KJ/mol. The liquid ammonia is collected as a final product. The unreacted N2 & H2 are recycled.

Le-Chatelier’s Principle in Ammonia Synthesis:
- Although, the reaction is exothermic in forward direction and thus, higher temperature will favour the backward going; but there is a need to provide higher temperature to break triple bond in N2 and a single bond in H2 to make a transition complex to gain energy of activation (Ea). The iron catalyst lower downs the energy barrier to speed up chemical reaction. After that, when the product ‘ammonia’ is formed, the excess amount of energy is released, making overall the exothermic. The energy released (r) is greater than the amount of it absorbed (a).

- The reactants have 4 moles overall and greater to 2 moles of product. So, to follow the Le-Chatelier’s principle, at higher pressure, the reaction will go forward according to Boyle’s law relationship. Further, the unreacted N2 & H2 gases are recycled to increase the concentrations of reactants, causing the reaction going forward. So, this all favours the production of ammonia with higher yield.
Safety Considerations:
A few the common safety considerations are given below:
- The synthesis of ammonia by Haber process is conducted at 200 atm, a very high pressure; so, the vessel is made with a thick-walled steel sheet to avoid any blast.
- At high pressure, the lower volume of gaseous products is safe by itself to avoid any explosion. Further pressure relief valves are installed to control the pressure at its level as per requirement.
- Gas leakage is controlled by air-tight system in the plant.
- The synthesis of ammonia takes place at 450° to 500°C, a very high temperature. So, cooling system is installed to avoid and control over heating. Further, installation of thermal insulation system is a key feature to avoid unnecessary heat lost and to maintain the temperature as per requirement.
- Hydrogen is highly flammable gas; so, no open spark or flame is allowed nearby around the plant.
- All the workers wear safety dresses to avoid any fire-catching, asphyxiation, eye or skin exposure to the atmosphere. Asphyxiation means a condition when there is a lack of oxygen and cause suffocation, ultimately leads to unconsciousness and even death.
Economic Considerations:
A few economic considerations are given below:
- The source of H2 gas is methane, a natural gas. So, it’s production is relatively not much costly.
- The source of N2 gas is air; so, it is also inexpensive.
- Unreacted gases are recycled to avoid wastage and reduce to production cost.
- The catalysts reduce the time and energy by speed up the reaction and by lowering energy of activation; so, reducing the cost.
- A large-scale production gives the yield relatively at lower cost.
Synthesis of Sulfuric Acid by Contact Process:
John Brown Francis Herreshoff (1850-1932) was an American chemist who developed contact process of sulfuric acid industrial production procedure in 1890. The chemical processing has the following procedure; described briefly:
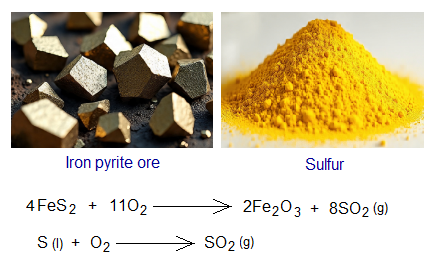
- First step is to produce sulfur dioxide (SO2). It is produced by burning a sulfide ore in air, like iron pyrite (FeS2: ferrous disulfide). This ore is gold like in its appearance and thus also called fool’s gold rock. It can also be produced by combusting molten sulfur in air. The sulfur melts at 112.8°C.
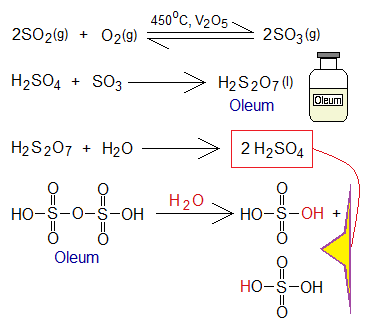
- Contact Tower: The SO2 gas and O2 from air are purified and preheated to about 450°C temperature and then feed into contact tower having the pressure 2 atom (202.65 kpa). There in tower, the gaseous mixture comes in contact with catalyst vanadium pentoxide (V2O5) present on shelves and form sulfur trioxide (SO3) gas.
- Absorption Tower: The SO3 gas is cooled and transferred to ‘absorption tower’; where concentrated H2SO4 is sprayed from the top. The SO3 is absorbed with acid and brings a chemical change to form oleum (pyrosulfuric acid or disulfuric acid). Oleum is a fumic colourless liquid with oil-like appearance. The ‘oleum’ is a Latin word meaning ‘oil or olive oil’. Then, oleum is allowed to react with water to form highly concentrated sulfuric acid.

Le-Chatelier’s Principle in SO3 Synthesis:
- Although, the reaction is exothermic in forward direction and thus, higher temperature will favour the backward direction; but there is a need to provide higher temperature to make a transition complex to gain energy of activation (Ea). The catalyst lower downs the energy barrier to speed up chemical reaction. After that, when the product SO3 is formed, the excess amount of energy is released, making overall the exothermic.
- The reactants (2SO2+O2) have 3 moles overall and greater to 2 moles of product (2SO3). So, to follow the Le-Chatelier’s principle, at higher pressure, the reaction will go forward according to Boyle’s law relationship.
Safety Considerations:
A few the common safety considerations are given below:
- The synthesis of sulfuric acid by contact process is conducted at 2 atm, a higher to normal 1 atm pressure; so, the thick-walled steel vessel is used.
- At higher pressure, the lower volume of gaseous product is safe by itself to avoid any explosion. Further pressure relief valves are installed to control the pressure at its level as per requirement.
- Gas leakage is controlled by air-tight system in the plant. Although, SO2 and SO3 are not flammable gases, but SO2 forms sulfurous acid (H2SO3) when dissolved in water; so, can be dissolved in moisture of skin, eyes. SO2 is irritating gas to respiratory tract, eyes, & skin; causing shortness of breath, coughing. SO2 can also cause asphyxiation by displacing O2 due to heavier than air. Likewise, SO3 can react with moisture to form sulfuric acid (H2SO4); which can cause severe burns of skin, eyes, and also corrode metals.
- The synthesis of SO3 takes place at 450°C, a very high temperature. So, cooling system is installed to avoid and control over heating. Further, installation of thermal insulation system is a key feature to avoid unnecessary heat lost and to maintain the temperature as per requirement.
- All the workers wear safety dresses to avoid any fire-catching, asphyxiation, eye or skin exposure to the atmosphere.
Economic Considerations:
Few are given below:
- The source of O2 gas is air; so, it is inexpensive.
- The use of catalysts reduces the time and energy by speed up the reaction and by lowering energy of activation; so, lowering the cost.
- A large-scale production with large-scale plants can reduce the cost, as compared to smaller plant.