Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Definitions, etymology of titration
- Concentration of a solution
- Procedure of titration
- Burette, conical flask
- Initial and final reading; end point
- Indicator
- Standard solution
- Molarity
- Titration formula
- Determining concentration: standardization
- Finding molarity of HCl solution: example 1
- Finding molarity of NaOH solution: example 2
- Finding molarity of sulfuric acid solution: example 3
- Determining composition by mass
- Finding for mixture the composition by masses, in grams and %; finding % purity and impurity; finding their amounts in grams in 250 cm3 of the solution: Example 4
- Learning the use of volumes in titration by exercise: exercise 2
Titration (Volumetric Analysis):
By definition,“the titration is a lab technique that is used to find out concentration of the substance in the solution”. Titration also known as ‘volumetric analysis’. The origin of the term ‘titration’ is from the old English ‘titre’ meaning ‘concentration of the substance’. The word ‘concentration’ means ‘the amount of the substance dissolved in a given volume of the solution’. Higher the amount, higher will be concentration.
Procedure of Titration:
Although, there are various types of titrations, but the main procedure is almost similar in all as described by acid-base type of titration.
- Acid solution is taken in burette and the initial reading of the tube is noted. Burette is a narrow glass tube, as shown below, with its volume calibration 0.0 ml to 50.0 ml.
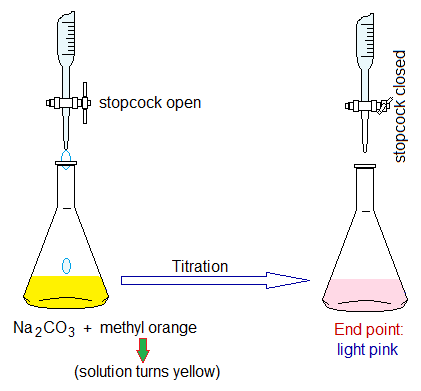
- The base solution is taken in conical flask and 1 or 2 drops of indicator are added. The indicator is an organic compound shows change in colour in acidic and basic media. Phenolphthalein is an example of such an indicator that is used with NaOH base. It gives pink colour in base and colourless in acid. So, the solution in flask turns pink. Methyl orange is another indicator that is used with Na2CO3 and NaHCO3 bases. It gives yellow colour with base; while light pink to red with acid.
- Titration: Now, drop-by-drop the acid solution is added into conical flask by careful opening of burette tap. The conical flask is whirled by hand after falling the drops.
- At a certain point, the pink solution of flask fades to light pink due to adding into it the burette (acid) solution. This is called end point; and at this point the final reading of the acid used is noted. At end point, one more drop can turn the light pink solution into colourless due to fully consumption of base (neutralized with acid: equivalent amount of acid and base) and the unreacted acid changes the solution into acidic media. The end point also called equivalence point or observable point.
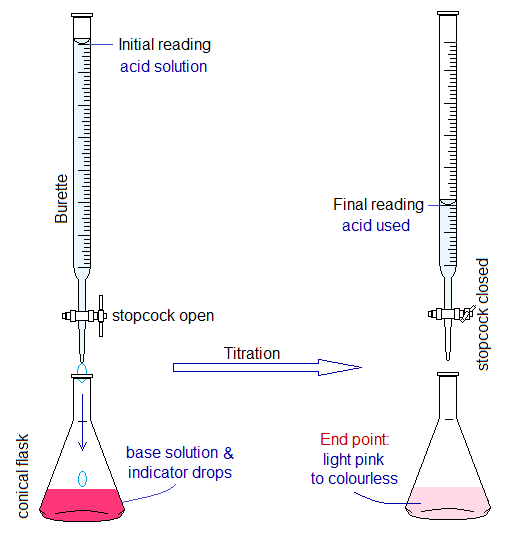
Standard Solution:
Out of two, one of the solutions is standard solution, might be an acid or a base. Standard solution is defined as, “the solution with known molarity”.
In titration, one solution is standard while the other has unknown molarity. So, with the help of standard solution, the unknown molarity is determined and this is titration.
Molarity:
It can be defined as, “the number of moles dissolved per dm3 of the solution”. Its formula is as under:
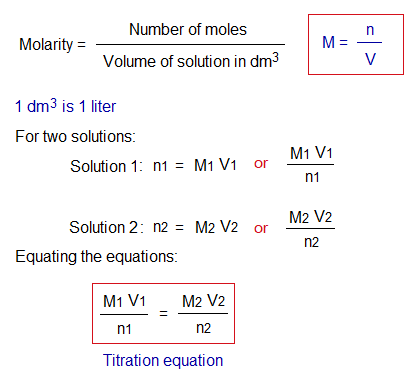
Determining Concentration: Standardization
To determine concentration of a solution by volumetric analysis is called standardization.
Example 1: Find out molarity of HCl solution by volumetric analysis. (In other words, to standardize the given solution of an acid against a standard solution of a base).
Standard solution (M2) = 0.1M NaOH
Indicator: Phenolphthalein (1 or 2 drops) in conical flask containing base. For the bases (alkalis: containing OH– ion) phenolphthalein indicator is used.
Molarity of HCl (M1) = ?
Balanced chemical equation & number of moles:
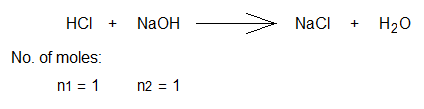
Volume of base solution taken in conical (titration, Erlenmeyer) flask (V2) = 10 cm3
Volume of acid used from burette for titration (V1) = 10 cm3
End point: Light pink to colourless
Following is the calculation that describes how the unknown molarity is determined by using titration formula.
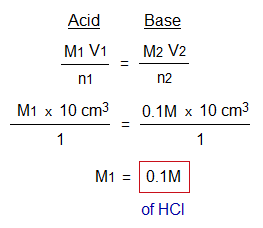
Further, after determining molarity, it is easy to find out amount (or strength) g/dm3.

Result: The concentration of HCl is 0.1M or 3.65 g/litre in terms of molarity or strength (amount) respectively.
Example 2: Find out molarity of NaOH solution by volumetric analysis.
Standard solution (M2) = 0.1M HCl
Molarity of NaOH (M1) = ?
Indicator: Phenolphthalein (1 or 2 drops) in conical flask containing base. Balanced chemical equation & number of moles:
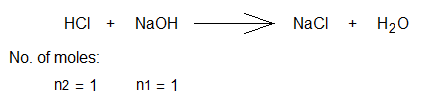
Volume of base solution taken in conical flask (V1) = 10 cm3
Volume of acid used from burette for titration (V2) = 9.5 cm3
End point: Light pink to colourless
Following is the calculation that describes how the unknown molarity and strength g/liter are determined by using titration formulae.
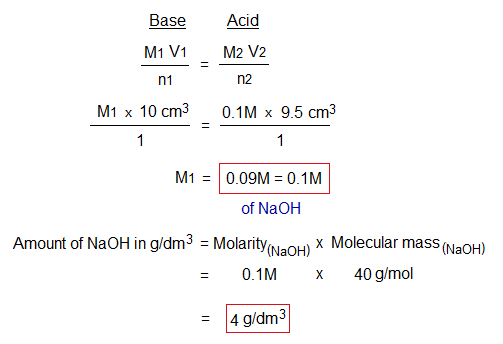
Result: The concentration of NaOH is 0.1M or 4 g/litre in terms of molarity or amount respectively.
Example 3: Find out molarity of sulfuric acid solution by volumetric analysis.
Standard solution (M1) = 0.1M NaOH
Indicator: Phenolphthalein (1 or 2 drops) in conical flask containing base.
Molarity of H2SO4 (M2) = ?
Volume of base solution taken in conical flask (V1) = 10 cm3
Volume of acid used from burette for titration (V2) = 9.5 cm3
End point: Light pink to colourless
Balanced chemical equation, number of moles, molarity and amount g/liter calculations are given below:
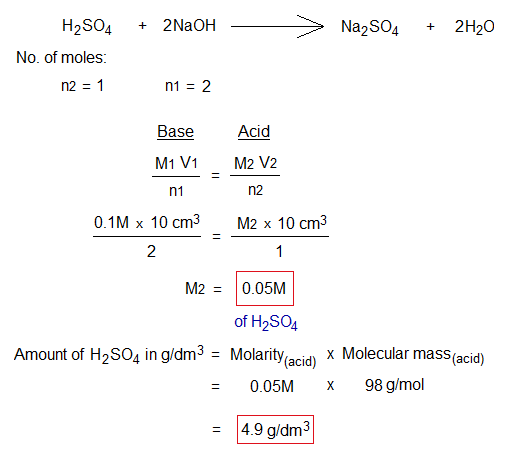
Exercise 1:
Standardize the given base solution by acid-base titration. Also, determine strength of base by g/dm3. Note: Standardization means to find out molarity. Use the following data.
Standard solution (M1) = 0.05M H2SO4
Molarity of NaOH (M2) = ?
Indicator: Phenolphthalein (1 or 2 drops) in conical flask containing base.
Volume of base solution taken in conical flask (V2) = 10 cm3
Volume of acid used from burette for titration (V1) = 10 cm3
End point: Light pink to colourless
Determining Composition by Mass
Example 4: Find out volumetrically:
- Composition by masses, in grams and in %, when 12g of a mixture containing sodium carbonate and sodium chloride salts are dissolved in an aqueous solution of 1 liter.
- Percentages of purity and impurity in the mixture.
- Also find out amounts Na2CO3 and NaCl in 250 cm3 of solution.
Note: In acid-base titration, the acid will react with Na2CO3; not with NaCl. So, sodium chloride will be considered impurity.
Standard solution (M2) = 0.1M H2SO4
Molarity of Na2CO3 (M1) = ?
Indicator: Methyl orange (1 or 2 drops) in conical flask containing Na2CO3 base solution. It turns yellow. For the bases of carbonates (CO32-) and bicarbonates (HCO3–) the methyl orange indicator is used.
Volume of base solution taken in conical flask (V1) = 10 cm3
Volume of acid used from burette for titration (V2) = 10 cm3
End point: Yellow turns light pink.
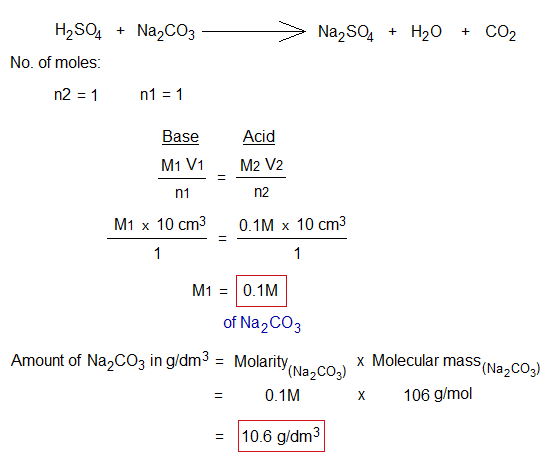
The mixture was taken 12g dissolved in 1 liter. Its composition by mass and percentages, can be determined mathematically by the following way:
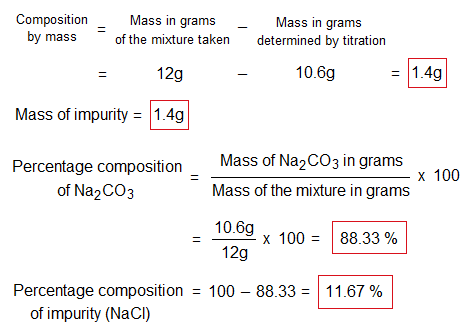
As the solution was prepared 1 liter (1 dm3) by dissolving a mixture of 12g. The amounts in 250 cm3 (or ml) of both the compounds will be calculated by the following way.

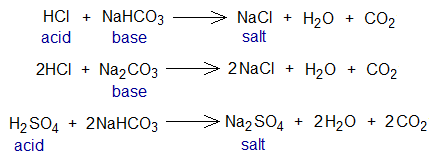
Exercise 2:
The following are a few other examples of methyl orange titrations. Both the acid and base solutions have their molarity 0.1M. Take 25 cm3 base solution into conical flask and find out the volume of acid that would be used to neutralize the base solution to the end-point (equivalence point) of the volumetric analysis.



I like this post, enjoyed this one thanks for posting.