Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Definition of Covalent Bond
- Lewis dot structures
- Features
- Electrons in OMS
- Key role of electron sharing
- Types of covalent bonds: single, double, triple
- Covalent bond in H2
- Covalent bond in Cl2
- Covalent bond in HCl
- Covalent bond in NH3
- Covalent bond in H2O
- Covalent bond in CH4
- Covalent bond in C2H4
- Covalent bond in O2
- Covalent bond in CO2
- Covalent bond in N2
Covalent Bond:
Definition: “The type of chemical bond that is formed by mutual sharing of electrons between atoms”.
This type of chemical bonding was proposed by an American chemistry professor Gilbert Newton Lewis (1875-1946) in 1916. He described the structures by dots called “Lewis Dot Structures”; as described below in the section of ‘electrons of OMS’.
Features:
- Unlike electrovalent bond, the ions are not formed. But the atoms fulfill their need of noble gas electronic configuration by mutual sharing of electrons in outermost shell (OMS).
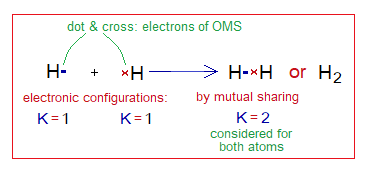
- The mutually shared electrons become the property of both the atoms and thus count for both the atoms. For example, hydrogen atom bears one electron in its K-shell. But there must be 2 according to noble gas electronic arrangement, as in helium. Two hydrogen atoms share their electrons together; thus, H2 molecule contains just two electrons in K-shell but mutually shared. So, each hydrogen atom can be said stable by helium electronic configuration.
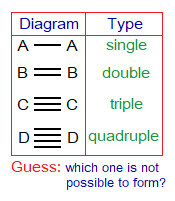
- Covalent bond has three types, single, double and triple. No above to triple exists.
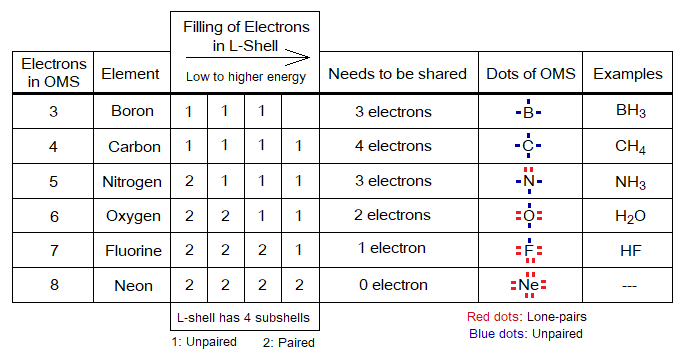
- The electrons exist in the form of pairs in sub-energy levels of the shells. The unpaired electrons of OMS need to be paired up and they do so by covalent sharing between atoms. Go through the following example of borane (BH3, aka boron trihydride).
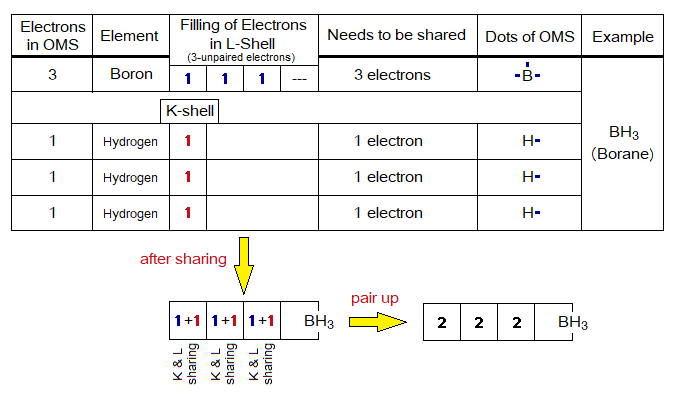
Electrons in OMS:
There might be two types of electrons in outermost shell, these are the following 1 & 2 (before sharing); and 1 & 3 (after sharing):
1. Lone pair (L.P.), aka non-bonding electrons. Because, these are paired, so don’t take part in covalent bond formation. It is not necessary that all atoms have L.P. of electrons. Noble gases have only these types of electrons.
2. Unpaired, aka single or not-paired electrons. These participate in covalent bond sharing to make pairs. Noble gases don’t have these electrons.
3. Bond pair, aka shared pair of electrons. The unpaired electrons when share to make pairs then these are called bond pairs.
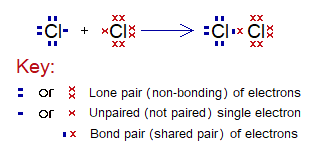
- The electrons exist in sub-energy levels of shells as pairs. The unpaired electrons pair up by sharing.
- In an atom, first, they like to be single while occupying the space in shell, then start pairing. In other words, distribute one-by-one in sub-energy levels of the shell, as shown in the following table. The L-shell has four pairs, total 8 electrons. Suppose, when there are 4 electrons to accommodate as in carbon, then they will not form two pairs but like to be single of 4 electrons. The 5th electron will make first pair as in nitrogen atom; and so on to the neon.
Can you guess why neon is a non-reactive element?
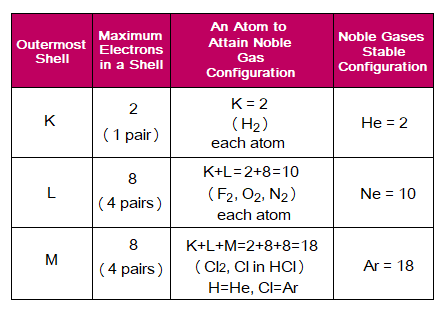
Key Role of Electron Sharing
Atoms need to establish stable electronic configuration of noble gases. So, they share electrons. The following table shows first two stable electronic configurations, i.e., of He and Ne, 2 and 10 respectively. If K-shell is the OMS, then it will try to attain 2 electrons. If L-shell is the OMS, it will try to attain 8 electrons, making K+L=10 electrons to get neon configuration.
Types of Covalent Bonds:
There are 3 types as stated above, single, double, and triple.
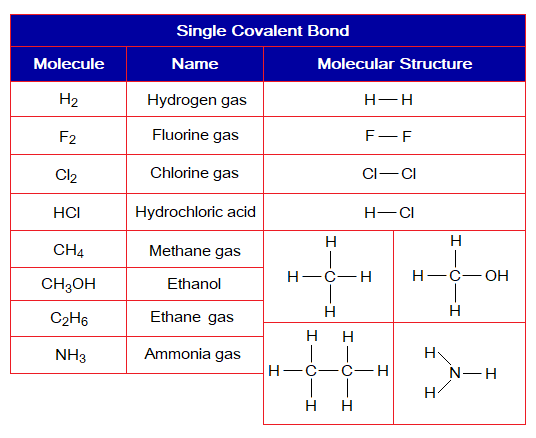
1. Single Covalent Bond: Definition: “The type of covalent bond that is formed by mutual sharing of two electrons between two atoms to make one shared pair of electrons”. It is denoted by a single line between atoms. Following examples form this type of chemical bonding.
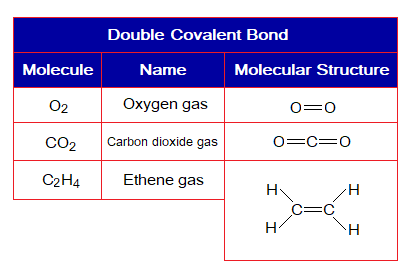
2. Double Covalent Bond: Definition: “The type of covalent bond that is formed by mutual sharing of four electrons in a total between two atoms to make two shared pair of electrons”. It is denoted by a double line between atoms. Following examples form this type of chemical bonding.
3. Triple Covalent Bond: Definition: “The type of covalent bond that is formed by mutual sharing of six electrons in a total between two atoms to make three shared pair of electrons”. It is denoted by a triple line between atoms. Following examples form this type of chemical bonding.
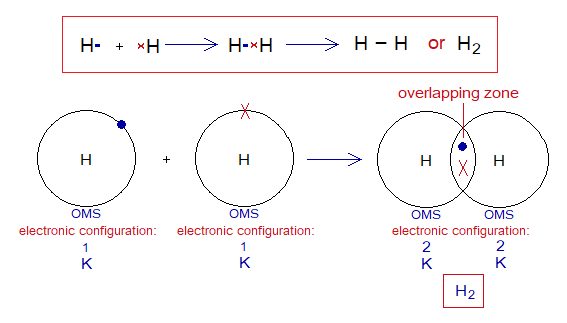
Covalent Bond in H2:
- Molecule: Hydrogen is a diatomic molecule formed by two hydrogen atoms.
- Type of bonding: One single covalent bond.
- OMS of H-atom: K-shellhaving one electron, and needs one more to pair up and attaining He electronic configuration.
- L.P and Single Electrons: No lone pair exists, but only a single electron exist that participates in sharing.
- Overlapping: Two shells of the hydrogen atoms overlap in the middle and resultantly sharing of electrons occur. The shared pair of electrons is mutually shared by both the atoms.
- Diagram: Dot and cross diagram of covalent sharing in H2 shown below without and with shells.
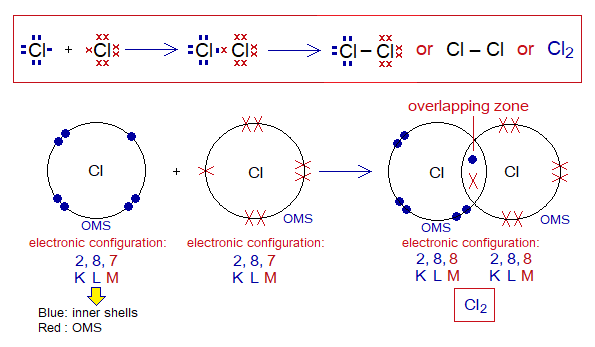
Covalent Bond in Cl2:
- Molecule: Chlorine is a diatomic molecule formed by two chlorine atoms.
- Type of bonding: One single covalent bond.
- OMS of Cl-atom: M-shellhaving 7 electrons, and needs one more to pair up and attaining argon electronic configuration of 18 electrons (K=2, L=8, M=8).
- L.P and Single Electrons: 3 lone pairs and 1 single electron exist. The unpaired electron participates in sharing.
- Overlapping: Two M-shells of two chlorine atoms overlap in the middle and resultantly sharing of electrons occur. The shared pair of electrons is mutually shared by both the atoms.
- Diagram: Dot and cross diagram of covalent sharing in Cl2 shown below without and with shells.
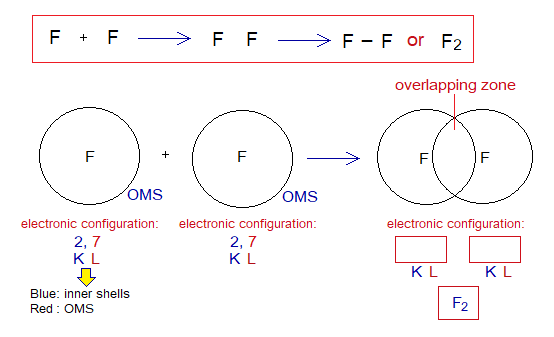
Exercise 1:
Describe the followings for fluorine molecule.
Covalent Bond in F2:
- Molecule: ______________________________________________
- Type of bonding: ________________________________________
- OMS of F-atom: ________________________________________
- L.P and Single Electrons: _________________________________
- Overlapping: ____________________________________________
- Diagram: Complete the following diagrams by dots and cross; and write in boxes the stable electronic configurations after sharing. To which noble gas the fluorine is following?
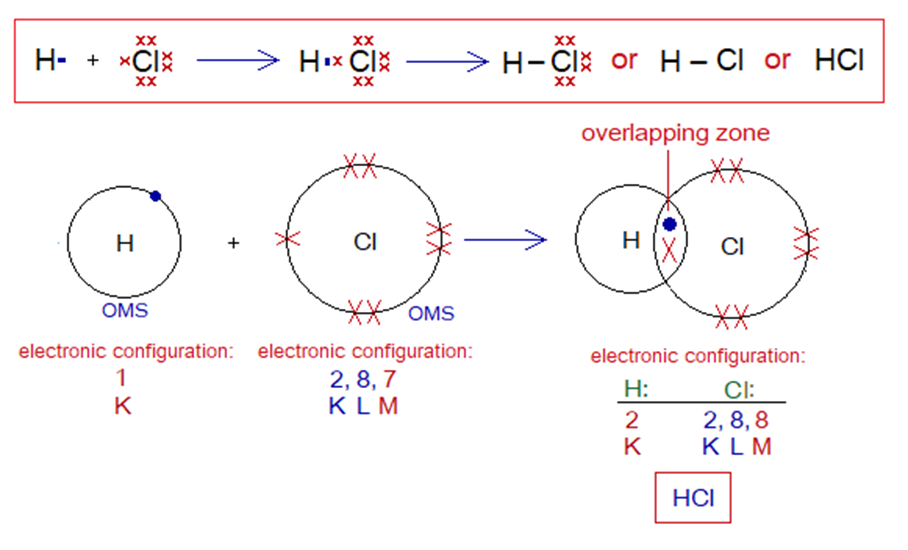
Covalent Bond in HCl:
- Molecule: Hydrochloric acid is a diatomic molecule formed by two non-identical atoms, hydrogen and chlorine.
- Type of bonding: One single covalent bond.
- OMS of Cl-atom: M-shellhaving 7 electrons, and needs one more to pair up and attaining argon electronic configuration of 18 electrons.
- OMS of H-atom: K-shellhaving one electron, and needs one more to pair up and attaining helium electronic configuration of two electrons.
- L.P and Single Electrons in Cl: 3 lone pairs and 1 single electron exist. The unpaired electron participates in sharing.
- L.P and Single Electrons in H: No lone pair exists, but only a single electron exist that participates in sharing.
- Overlapping: The K-shell of hydrogen and M-shell of chlorine atom overlap together in the middle and resultantly sharing of electrons occur. The shared pair of electrons is mutually shared by both the atoms.
- Diagram: Dot and cross diagram of covalent sharing in HCl shown below without and with shells.
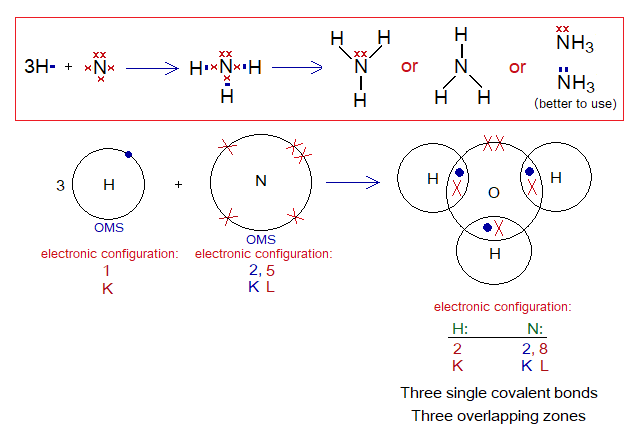
Covalent Bond in NH3:
- Molecule: Ammonia is a tetra-atomic molecule formed by non-identical atoms; 3 hydrogen and 1 nitrogen atom mutually share at three different overlapping zones.
- Type of bonding: Three single covalent bonds.
- OMS of N-atom: L-shellhaving 5 electrons, and needs 3 more to pair up and attaining neon electronic configuration of 10 electrons.
- OMS of H-atom: K-shellhaving one electron, and needs one more to pair up and attaining helium electronic configuration of two electrons.
- L.P and Single Electrons in N: 1 lone pair and 3 unpaired electrons exist. The unpaired electrons participate in sharing.
- L.P and Single Electrons in H: No lone pair exists, but only a single electron exist that participates in sharing.
- Overlapping: The K-shell of one hydrogen and L-shell of nitrogen atom overlap together and resultantly sharing of electrons occur. Likewise, two more similar overlappings occur to form ammonia molecule. 1 nitrogen overlaps with 3 hydrogen atoms and makes three single covalent bonds.
- Diagram: Dot and cross diagram of covalent sharing in NH3 shown below without and with shells.
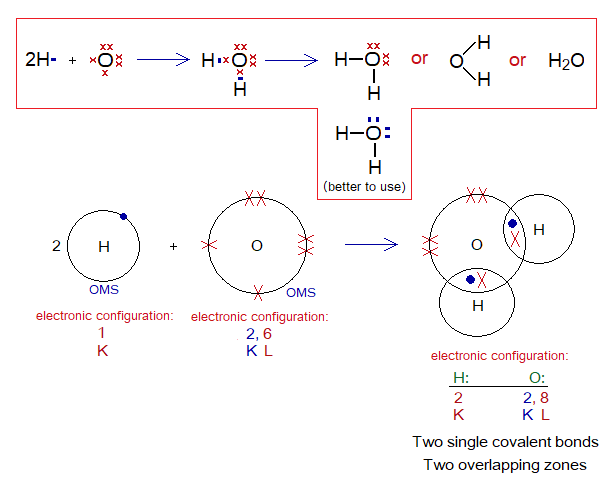
Covalent Bond in H2O:
- Molecule: Water is a triatomic molecule formed by non-identical atoms; 2 hydrogen and 1 oxygen atoms mutually share at two different overlapping zones.
- Type of bonding: Two single covalent bonds.
- OMS of O-atom: L-shellhaving 6 electrons, and needs 2 more to pair up and attaining neon electronic configuration of 10 electrons.
- OMS of H-atom: K-shellhaving one electron, and needs one more to pair up and attaining helium electronic configuration of two electrons.
- L.P and Single Electrons in O: 2 lone pairs and 2 unpaired electrons exist. The unpaired electrons participate in sharing.
- L.P and Single Electrons in H: No lone pair exists, but only a single electron exist that participates in sharing.
- Overlapping: The K-shell of one hydrogen and L-shell of oxygen atom overlap together and resultantly sharing of electrons occur. Likewise, one more similar overlapping occurs to form water molecule. 1 oxygen overlaps with 2 hydrogen atoms and makes two single covalent bonds.
- Diagram: Dot and cross diagram of covalent sharing in H2O shown below without and with shells.
Covalent Bond in CH4:
- Molecule: Methane is a penta-atomic molecule formed by non-identical atoms; 4 hydrogen and 1 carbon atoms mutually share at four different overlapping zones.
- Type of bonding: Four single covalent bonds.
- OMS of C-atom: L-shellhaving 4 electrons, and need 4 more to pair up and attaining neon electronic configuration of 10 electrons.
- OMS of H-atom: K-shellhaving one electron, and needs one more to pair up and attaining helium electronic configuration of two electrons.
- L.P and Single Electrons in C: No lone pair but 4 unpaired electrons exist. The unpaired electrons participate in sharing.
- L.P and Single Electrons in H: No lone pair exists, but only a single electron exist that participates in sharing.
- Overlapping: The K-shell of one hydrogen and L-shell of carbon atom overlap together and resultantly sharing of electrons occur. Likewise, three more similar overlappings occur to form methane molecule. 1 carbon overlaps with 4 hydrogen atoms and makes four single covalent bonds.
- Diagram: Dot and cross diagram of covalent sharing in CH4 shown below without and with shells.

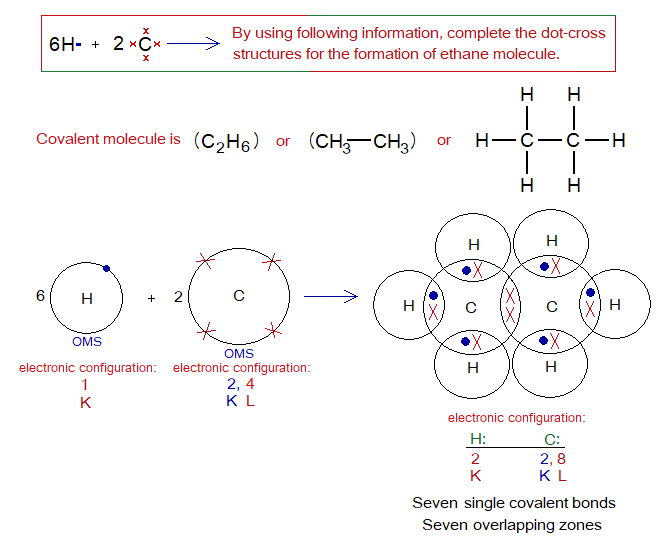
Exercise 2:
Describe the followings for ethane molecule.
Covalent Bond in C2H6:
- Molecule: _______________________________________________
- Type of bonding: _________________________________________
- OMS of C-atom: __________________________________________
- OMS of H-atom: __________________________________________
- L.P and Single Electrons in C: ______________________________
- L.P and Single Electrons in H: ______________________________
- Overlapping: ____________________________________________
Exercise 3:
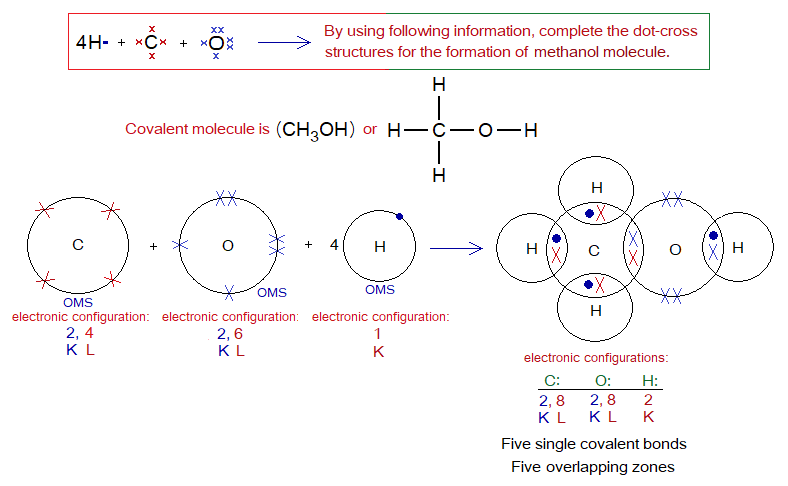
Describe the followings for methanol (methyl alcohol) molecule.
Covalent Bond in CH3OH:
- Molecule: _______________________________________________
- Type of bonding: __________________________________________
- OMS of C-atom: ___________________________________________
- OMS of O-atom: ___________________________________________
- OMS of H-atom: ___________________________________________
- L.P and Single Electrons in C: ______________________________
- L.P and Single Electrons in O: ______________________________
- L.P and Single Electrons in H: ______________________________
- Overlapping: _______________________________________________
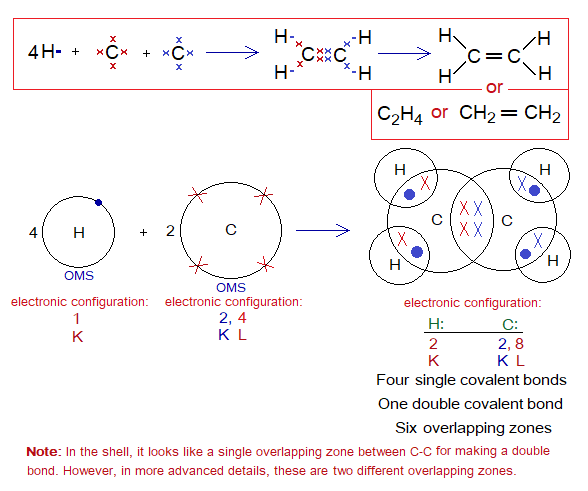
Covalent Bond in C2H4:
- Molecule: Ethene is a hexa-atomic molecule formed by identical and non-identical atoms; 4 hydrogen and 2 carbon atoms mutually share at six different overlapping zones.
- Type of bonding: Four single covalent bonds between hydrogens and carbon; a double covalent bond between both carbon atoms.
- OMS of C-atom: L-shellhaving 4 electrons, and need 4 more to pair up and attaining neon electronic configuration of 10 electrons.
- OMS of H-atom: K-shellhaving one electron, and needs one more to pair up and attaining helium electronic configuration of two electrons.
- L.P and Single Electrons in C: No lone pair but 4 unpaired electrons exist. The unpaired electrons participate in sharing.
- L.P and Single Electrons in H: No lone pair exists, but only a single electron exist that participates in sharing.
- Overlapping: Two hydrogen atoms share with a carbon atom by K-L overlapping at two overlapping zones. Likewise, two more hydrogen atoms share with another carbon by K-L overlappings. The two carbons mutually share by L-L overlapping at two overlapping zones and make a C-C double bond.
- Diagram: Dot and cross diagram of covalent sharing in C2H4 shown below without and with shells.
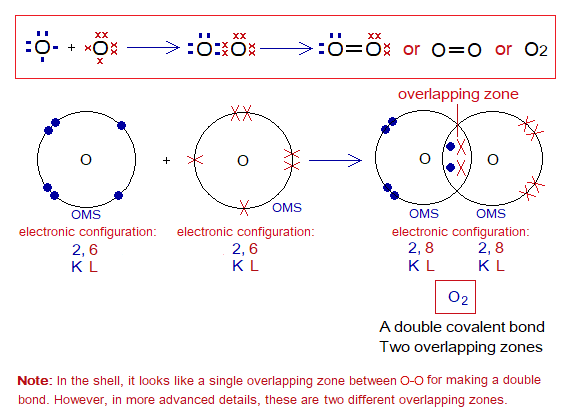
Covalent Bond in O2:
- Molecule: Oxygen is a diatomic molecule formed by two identical atoms.
- Type of bonding: One double covalent bond between two oxygen atoms.
- OMS of O-atom: L-shellhaving 6 electrons, and needs 2 more to pair up and attaining neon electronic configuration of 10 electrons.
- L.P and Single Electrons in O: 2 lone pairs and 2 unpaired electrons exist. The unpaired electrons participate in sharing.
- Overlapping: Two oxygen atoms share by L-L overlapping at two overlapping zones and make a double bond between atoms.
- Diagram: Dot and cross diagram of covalent sharing in O2 shown below without and with shells.
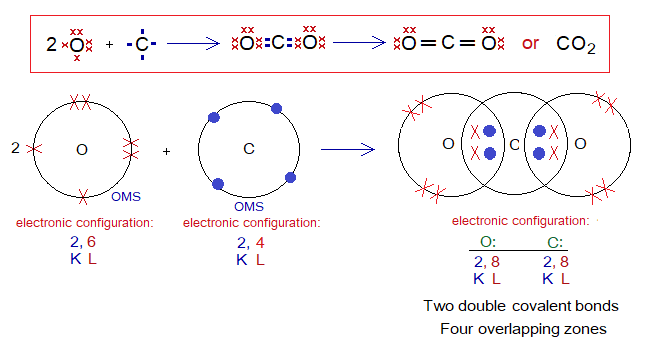
Covalent Bond in CO2:
- Molecule: Carbon dioxide is a triatomic molecule formed by three atoms.
- Type of bonding: Two double covalent bonds between a carbon atom and two oxygen atoms.
- OMS of C-atom: L-shellhaving 4 electrons, and need 4 more to pair up and attaining neon electronic configuration of 10 electrons.
- OMS of O-atom: L-shellhaving 6 electrons, and needs 2 more to pair up and attaining neon electronic configuration of 10 electrons.
- L.P and Single Electrons in C: No lone pair but 4 unpaired electrons exist. The unpaired electrons participate in sharing.
- L.P and Single Electrons in O: 2 lone pairs and 2 unpaired electrons exist. The unpaired electrons participate in sharing.
- Overlapping: Two oxygen atoms share with a single carbon atom by L-L overlapping at two different overlapping zones and make a two C-O double bonds.
- Diagram: Dot and cross diagram of covalent sharing in CO2 shown below without and with shells.
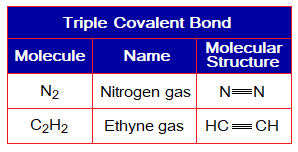
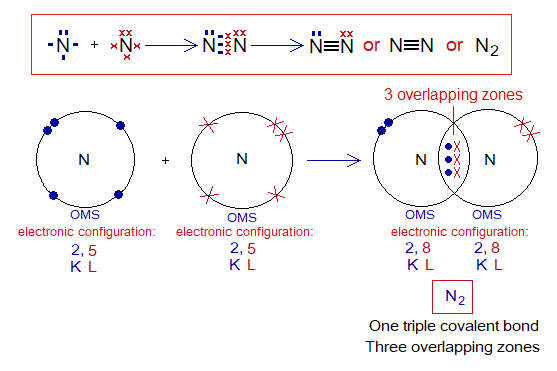
Covalent Bond in N2:
- Molecule: Nitrogen is a diatomic molecule formed by two identical atoms.
- Type of bonding: One triple covalent bond between two nitrogen atoms.
- OMS of N-atom: L-shellhaving 5 electrons, and needs 3 more to pair up and attaining neon electronic configuration of 10 electrons.
- L.P and Single Electrons in N: 1 lone pair and 3 unpaired electrons exist. The unpaired electrons participate in sharing.
- Overlapping: Two nitrogen atoms share by L-L overlapping at three overlapping zones and make a N-N triple bond.
- Diagram: Dot and cross diagram of covalent sharing in N2 shown below without and with shells.
Exercise 4:
Describe in detail the covalent bonding in ethyne molecule (C2H2); support your answer with respect to OMS electronic configurations, change in configurations according to noble gases by covalent sharing, dot-cross structures with and without shells, L.P. (if exist) and unpaired electrons. The ethyne molecule is HCΞCH. There is formed a triple bond between two carbon atoms. Other name of ethyne is acetylene.



