Dr. Mudassar Altaf, Associate Professor, Higher Education Department, Govt. of the Punjab, Pakistan.
The copy of the content is not allowed
Contents:
- Introduction
- Mechanism of Synthesis
- Base catalyzed
- Acid catalyzed
Introduction:
Two ketone molecules show condensation together on the mechanism similar to aldol synthesis. Ketone molecules might be similar or dissimilar. As a result of condensation, the beta-hydroxy ketone is formed.
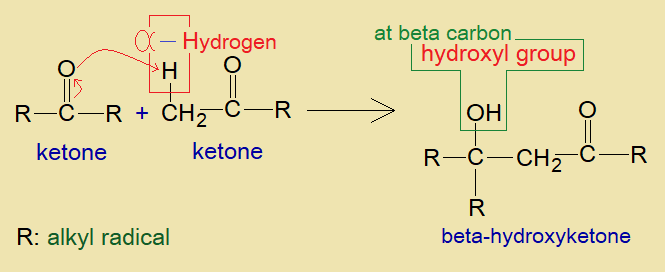
Mechanism of the Synthesis:
It is an aldol condensation reaction between two ketones. If these are similar then it would be self-condensation. Like aldol condensation, the presence of alpha-hydrogen is mandatory. The mechanism is either base catalyzed or acid catalyzed.
a. Base Catalyzed:
The base (OH–) makes water with acidic alpha-hydrogen of a ketone molecule. Thus, the alpha-carbon makes carbanion (active methylene), which attacks on partial positive carbonyl-carbon of another ketone; and carbon-carbon linkage is developed. The corresponding oxygen forms oxyanion (oxoanion) on beta carbon that ultimately changes to hydroxyl group as revealed in the mechanism. The, hydroxide ion of the alkali is formed at the end. When there is self-condensation in propanone, then, 4-Hydroxy-4-methyl-2-pentanone is formed, as shown in the chemical change.
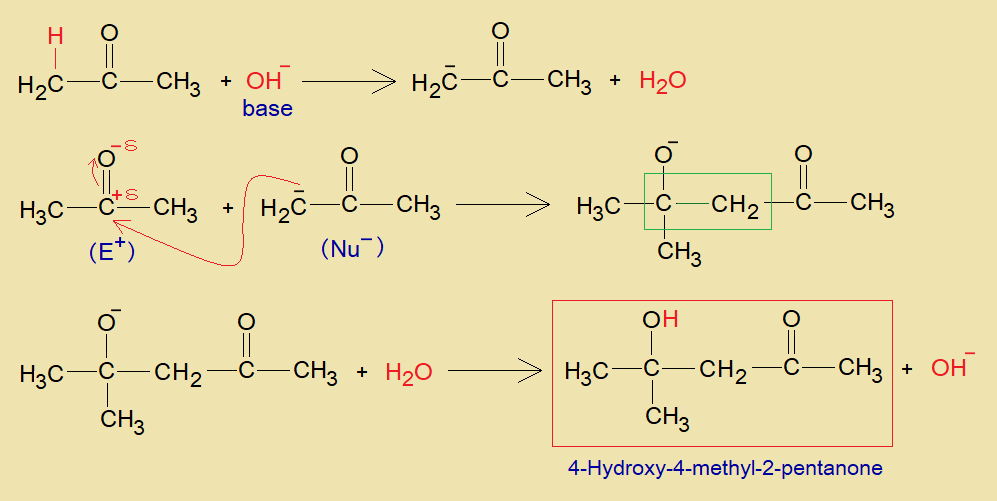
b. Acid Catalyzed:
The H+ of an acid forms hydroxyl group with carbonyl oxygen and thus, makes carbocation at ketone. Thereafter, this positively charged carbon develops a carbon-carbon linkage with partial negative alpha-carbon of another ketone and yields the final product, beta-Hydroxyketone. The alpha-hydrogen is removed as H+ ultimately, as revealed in the mechanism below.
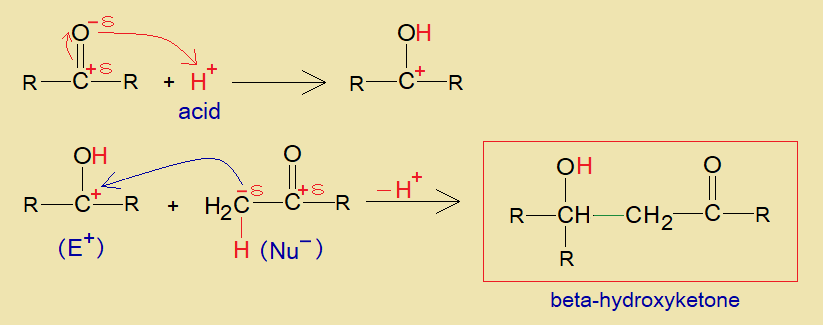
Ketones and formaldehyde also form beta-Hydroxyketone by aldol condensation.



