Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Physical change: definition and explanation
- Chemical change: definition and explanation
- Collision theory: definition, successful collision, four factors affecting including energy of activation
- Rate of reaction: definition, formula, graph, five factors affecting including catalysts/enzymes
- Calculations based upon rate of reaction
Physical Change:
Definition: “The change in the substance in its appearance, shape and size without change in chemical composition and without formation of a new product is called physical change”.
For example, water appears in three states (phases), solid in the form of ice, liquid, and gas in the form of steam, and water vapours. The composition remains H2O in all, but the appearances, shapes and sizes are different. The phase change needs change in heat energy. The ice needs heat to melt and liquid again needs heat to convert into gaseous state; the opposite needs cooling. It is a reversible change and can easily be reversed.
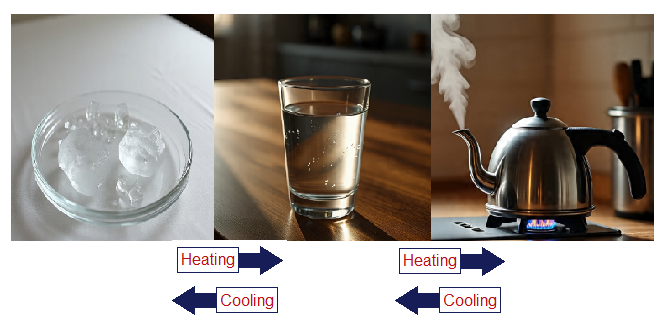
Chemical Change:
Definition: “The change in the substance in its chemical composition by the formation of a new product(s) is called chemical change”.
For example, water molecules split into hydrogen and oxygen gases by means of chemical change. Hydrogen (H2) and oxygen (O2) are different compounds and are gases; while water is another compound and is liquid at room temperature. Not all chemical changes are reversible; many are irreversible.
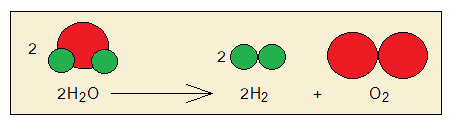
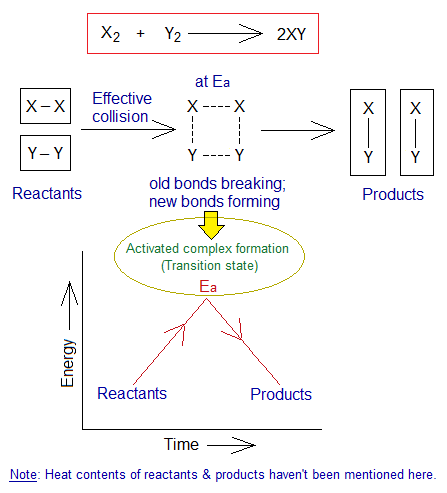
Collision Theory:
Definition: This theory states that, “for a chemical reaction to occur, the particles of the reactants collide with one another with enough energy in right direction, so that new product(s) are formed”. This theory was proposed in 1916 by a German Chemist Max Trautz (1880-1960); and in 1918 by a British chemist William C. M. Lewis (1885-1956). They worked independently.
The collision must be effective to form new products is called successful collision. For a successful collision, the following factors play their role.
- Number of particles participating in collision per unit of volume of the system.
- Frequency of hitting per unit time.
- Kinetic energy of the particles hitting each other.
- The activation energy (Ea).
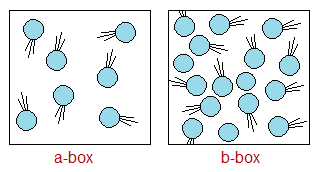
Number of Particles Per Unit Volume: More the number of particles per unit volume participating in collision, more will it be successful. Judge from the diagram for ‘a’ versus ‘b-box’ where is the more successful collision?
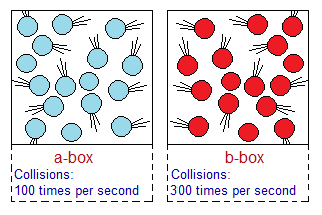
Frequency of Hitting Per Unit Time: More collisions are taking place in a unit time; more will it be successful. Judge from the diagram for ‘a’ versus ‘b-box’ where is the collision frequent? Each particle collides billions of times per second. In a real sense, they don’t hit one another like solid balls of billiard game. But, due to electrostatic forces of attraction and repulsion, they repel each other from a certain distance. This distance depends upon temperature and applied pressure. This pull and push force is called collision to provide us an understanding in a certain sense. So, it might be not a wrong to say that, it is a hypothetical collision.
Kinetic Energy (K.E.) of the Particles: As it is well known that the ‘kinetic’ has its Greek origin from the word ‘kinesis’ meaning ‘movement’. So, the energy of the particles due to their motion is called kinetic energy. The movements of particles cause them to collide with one another. The collisions make them to move faster and faster and hence, the K.Es. of particles increase subsequently. This leads to break old bonds and put the system into transition phase, i.e., high energy state called activation energy.
Energy of Activation (Ea): The Ea is an energy barrier, a higher energy state called transition energy where old bonds break and new are formed; in other words, reactants are going to be changed into products. So, an activated complex is formed at this stage. Detailed discussion is given in the link https://chemiologist.com/chemical-energetics/
Rate of Reaction:
Definition: The rate means ‘speed’ by which the reaction occurs to make products with respect to time calculations. It can be stated as, “The increase in concentration of products in a unit time by decreasing the concentration of reactants” is called rate of a chemical reaction. In a unit time, more product formation portrays the rate high; in other words, more consumption of reactants.
If C is the concentration of the reactants or products, ∆t is the change of time, then, it would be expressed mathematically as:
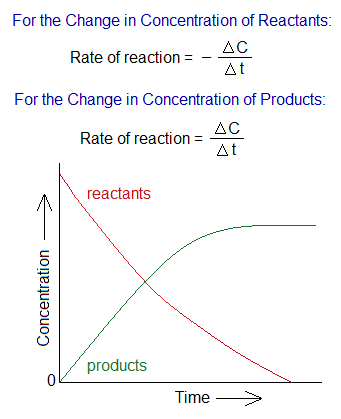
In the above relationship, negative ∆C indicates decrease in concentration of the reactants, as red graph line shows. In the second relationship, positive ∆C indicates increase in concentration of the products, as green graph line elaborates; and the line becomes straight along the time, means no more concentration is increasing due to fully consumption of reactants.
Factors Affecting Rate of Chemical Reaction
The following are the factors affecting the rate of chemical reactions.
- Changing in concentration of the solution
- Changing the pressure of the gases
- Changing the surface area of the solids
- Changing the temperature of the system
- Effect of catalysts or enzymes
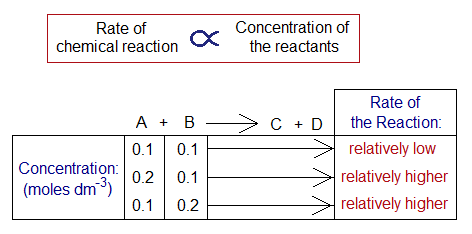
1. Changing in Concentration of the Solution: By increasing the concentration of the reactants, the rate will also be increased and vice versa. There is a direct relationship of rate of chemical reaction with the concentration of the reactants. Suppose, in a reaction between A & B forming C & D, the initial concentrations of both A and B were 0.1 molar. But, when the concentration of A was increased to 0.2 moles, the rate was increased. Similarly, by increasing the concentration of B, the speed of the reaction was also increased.
2. Changing the Pressure of Gaseous System: The normal (standard) pressure is 1 atmospheric (atm). If the pressure of the system is increased, then the frequency of collisions between reactant particles also increases. Consequently, rate of the reaction goes up. Haber process is used for the synthesis of ammonia, carried out at very high pressure of 200 atm. Go to the link for details https://chemiologist.com/chemistry-of-reversible-reactions-and-their-industrial-application/
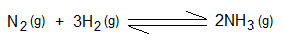
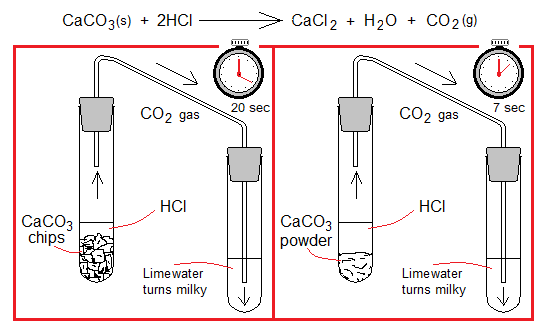
3. Changing the Surface Area of Solids: The fine powder of a substance has larger surface area as compared to its large crystals, chips, grains, lumps or chunks. Fine powdered form will react faster. For example, take two test tubes and add to one powdered CaCO3 and to the other its larger chips. Now, add in both dilute HCl. The chemical reaction in both will be same, but will be faster having powdered CaCO3. The CO2 turns lime water [Ca(OH)2] milky when passed through it due to the formation of CaCO3; and will be quicker in the system containing powdered calcium carbonate as compared to the other.
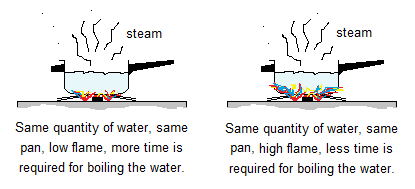
4. Changing the Temperature of the System: Rise in temperature increases the kinetic energies of the particles, causing the increase in frequency of collision per unit time. Thus, resultantly, the rate of chemical reaction also increases. It is a general observation that water boils slow when placed on low flame; while boils quickly if the flame of the stove is turned up.
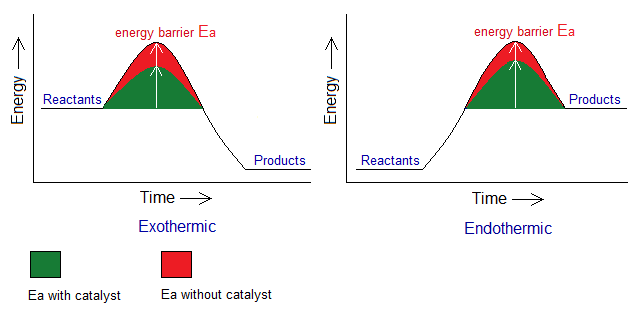
5. Effect of catalysts or enzymes: By definition, “catalysts are the substances that speed up the chemical reactions”. These are used in laboratories as well as in industries. The enzymes are “the biological catalysts”. For example, ‘salivary amylase’ is an enzyme in our saliva that breaks down complex carbohydrates into simple sugar molecules. The catalysts reduce the energy of activation (Ea), i.e., lowering the energy barrier; so, rate of the reaction goes up. Following two graphs are given to explain the role of a catalyst/enzyme in increasing the rate of a chemical reaction (occurs more quickly).
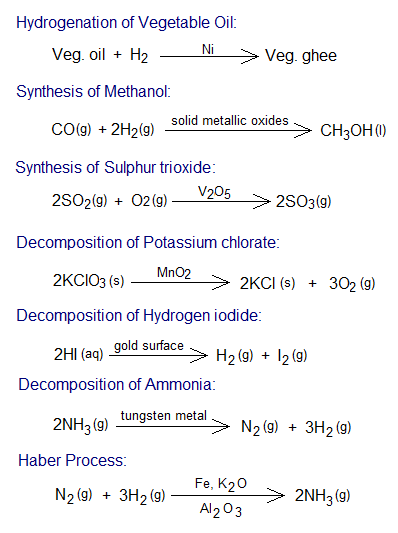
Following few examples are given for various chemical reactions and their catalysts:
- The vegetable oils (liquids) are hydrogenated into vegetable ghee (solids) in the presence of nickel catalyst.
- Solid metallic oxides are used as catalysts for the synthesis of methyl alcohol from water gas (mixture of carbon monoxide and hydrogen gases).
- A catalyst, vanadium pentoxide (V2O5) a yellowish-red powder is used for the synthesis of sulphur trioxide (SO3) from sulphur dioxide (SO2) and oxygen gases during industrial method called ‘contact process’ for the synthesis of H2SO4.
- Potassium chlorate (KClO3) uses manganese dioxide (MnO2) as a catalyst to decompose into potassium chloride (KCl) and oxygen gas.
- On the surface of gold (acts as a catalyst), the hydrogen iodide is decomposed to generate hydrogen and iodine gases.
- Tungsten metal (W) is used as a catalyst for the decomposition of ammonia into nitrogen and hydrogen gases.
- Haber process is used for the synthesis of ammonia gas in the presence of iron (as primary catalyst) along with potassium oxide (K2O) and aluminum oxide (Al2O3).
- The enzyme detergents are also used in laundry (washing clothes) to remove strains. For example, protein strains are removed by the enzymes ‘proteases’. To remove fat strains, the enzymes ‘lipases’ are used; and for the removal of carbohydrates’ strains, the enzymes ‘amylases’ are used.
- Amylase, pepsin and lipase are enzymes in human body to breakdown complex carbohydrates into simple sugar, proteins into peptides, and fats into fatty acids and glycerol respectively.
- During fermentation, the enzymes from microorganisms are used. For example, to make yogurt, the bacteria ‘Lactobacillus’ produces the enzyme ‘lactase’ to break down lactose (milk sugar) into galactose and glucose; then further, bacteria utilize them to make lactic acid.
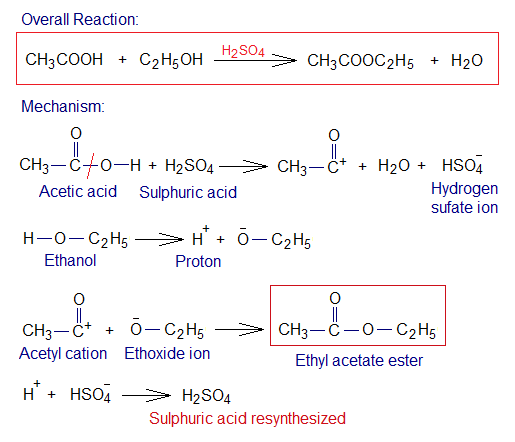
Another important aspect of the catalysts is that they remain unchanged at the end of the reaction; in other words, consumed but regenerated (resynthesized, reappeared). In the following example, a simple mechanism has been given to show resynthesis of sulphuric acid catalyst. Although, the detailed mechanism is somewhat complicated; but a general concept has been elaborated. This is a chemical reaction between acetic acid and ethanol to synthesize ethyl-acetate ester and water in the presence of H2SO4 catalyst. This reaction is called esterification.
Calculations Based Upon Rate of Reaction:
Magnesium ribbon reacts with dilute HCl to produce MgCl2 and H2 gas. The rate of the chemical reaction depends upon:
- the thickness of the magnesium ribbon. More, thin it will be, higher would be the reaction rate.
- the concentration of HCl aqueous solution. As an example, the reaction rate will be higher with 1 molar solution as compared to 0.1 molar.
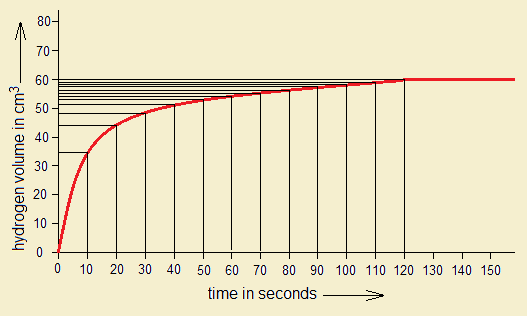
1. By the following graph, if 60 cm3 hydrogen gas is produced in 120 seconds, (1) calculate the amount of Mg consumed in grams. (2) If HCl is 1 molar, then, for 60 cm3 H2 production, the required amount of Mg will react with how many grams and moles of HCl to that time?
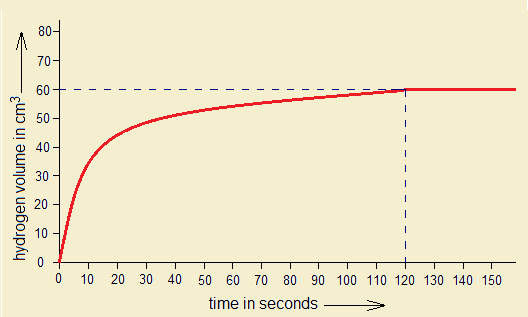
Following chemical equation and calculations will help to solve the problem.
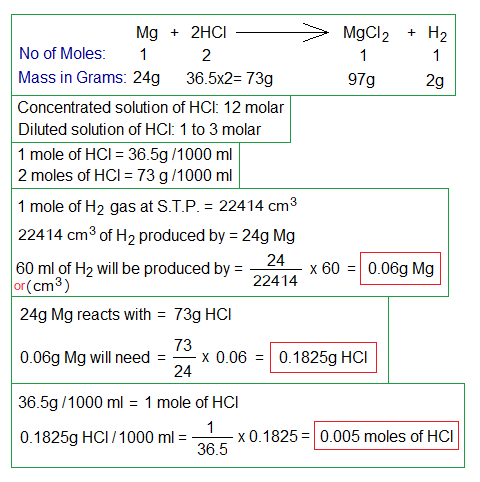
- The standard chemical equation describes that 1 mole (24g) of Mg reacts with 2 moles (73g) of HCl to produce 1 mole (97g) of MgCl2 and 1 mole (2g) of H2 gas.
- The standard concentrated solution of HCl as lab reagent is 12 molars (438g per 1 dm3 of aqueous solution). As 1000 ml = 1000 cm3 = 1 dm3 = 1 liter.
- The diluted HCl solution in lab is 1 to 3 moles. 1 mole contains 36.5g per dm3.
- 1 mole of any gas occupies 22414 cm3 volume at standard temperature & pressure (S.T.P.). So, 60 cm3 of H2 gas will be produced by 0.06g of Mg, as calculated above.
- 0.06g of Mg will react with 0.1825g of HCl (0.005 moles per dm3) to that time (120 seconds).
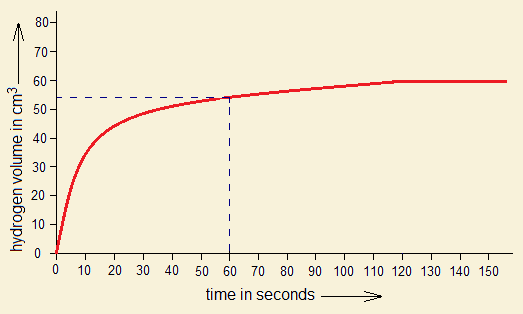
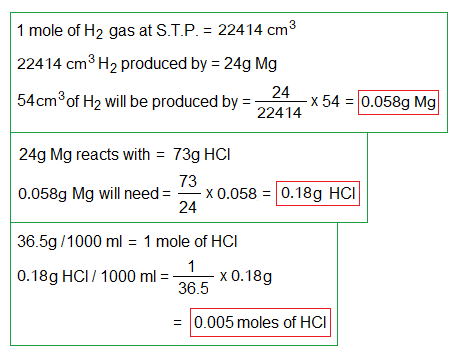
2. By the following graph, if 54 cm3 gas is produced in 60 seconds, (1) calculate the amount of Mg consumed in grams. (2) If HCl is 1 molar, then, for 54 cm3 H2 production, the required amount of Mg will react with how many grams and moles of HCl to that time?
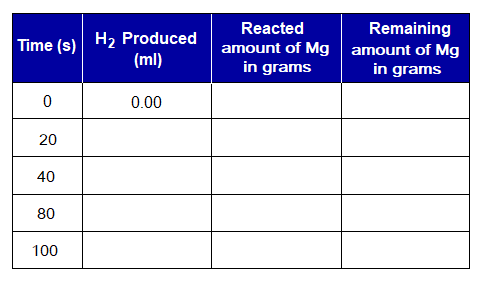
Exercise 1:
If 12g of Mg are taken to react with 1 molar HCl solution to produce magnesium chloride and hydrogen gas. For the rate of chemical reaction, the formation of hydrogen gas measured in cm3 with respect to time in seconds; and the following graph was made. (1) Use the graph to fill-in the boxes of the table by the amount of hydrogen gas produced at various times. (2) Also calculate the amount of Mg in grams used at a particular time and further, how much would be the remaining amount of magnesium at that time.