Dr. Mudassar Altaf, Associate Professor, Higher Education Department, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Why Kinetic Particle Theory?
- Definition of KPT
- Particles’ separation, arrangement & motion in solids
- Particles’ separation, arrangement & motion in liquids
- Particles’ separation, arrangement & motion in gases
Why Kinetic Particle Theory?
Kinetic particle theory (KPT) is a model that explains movements of particles in different states of the matter. KPT aka Kinetic Theory of Matter (KTM). The term ‘kinetic’ has its root from Greek word ‘kinein’ meaning ‘to move’.
Definition of KPT:
This theory deals with the physical properties of the matter related to arrangement and motion of the particles.
The three states of the matter have their distinguished characteristics related to separation, arrangement and the motion of the particles as described below.
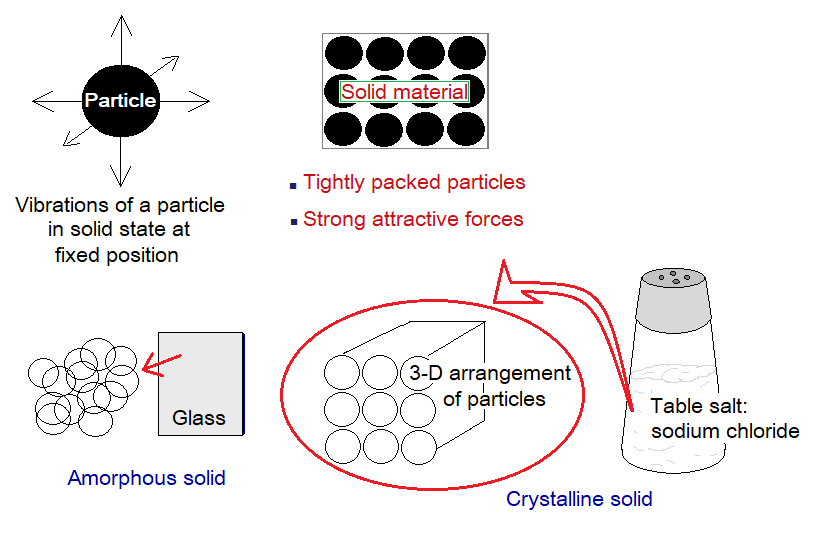
Solids:
- Packing: The particles in solids are tightly packed. The arrangement might be regular in three-dimensional repeating pattern, as in crystals; or lacking regular 3D-repeating pattern, as in amorphous solids. The ‘morphe’ is a Geek word, meaning ‘shape’; and ‘a’ for ‘without’. The example of crystalline solid is ‘sodium chloride’, and the example of amorphous solid is ‘glass’.
- Attractive Forces: The particles in solid state have very strong attractive forces with one another. Unlike liquids and gases, they cannot change their positions.
- Motion: The particles cannot move from their fixed positions; however, can vibrate back and forth.
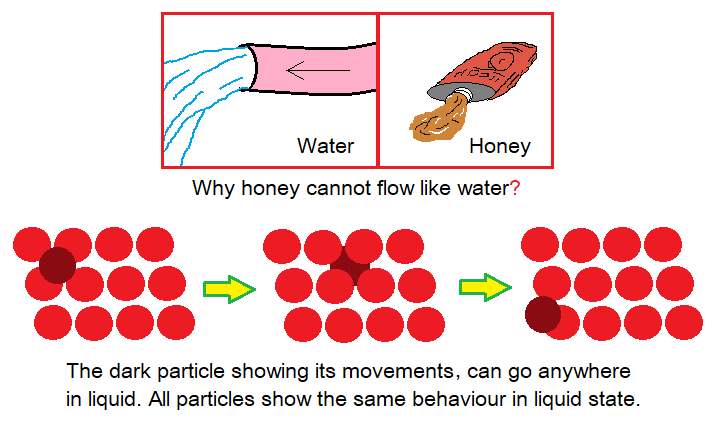
Liquids:
- Packing: The particles in liquids are relatively less tightly packed than solids; and the space between them is relatively larger as compared to solids. Further, there is no regular arrangement of particles exist.
- Attractive Forces: The attractive forces are relatively less strong as compared to solids. So, particles are free to move and are not bounded to be fixed at their positions.
- Motion: Liquids exhibit property to flow. Different liquids have different rates of flow at same temperature, like honey is thick and its flow is too much slow as compared to water.
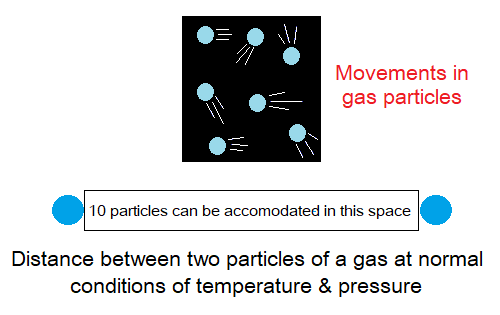
Gases:
- Packing: The particles in gases are far away from one another. There is enough space between two particles to hold more or less ten particles.
- Attractive Forces: Gases have least attractive forces between particles. It is a false concept that there is no attractive forces between gas particles.
- Motion: The particles move fast. Their mobility is random (haphazard). A particle moves straight unless collides with another one, like pawns (coins) of a carrom board.