Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents
Introduction
- Physical Properties
- Solution Preparation in Lab.
- Methyl orange & PH
- Titration
- Synthesis of methyl orange compound
Introduction:
Methyl orange is an organic compound that is used in acid-base titration. It shows different colours in acidic and basic media; it is yellow in basic but red in acidic medium. This is the reason why methyl orange is used as indicator in volumetric analysis.
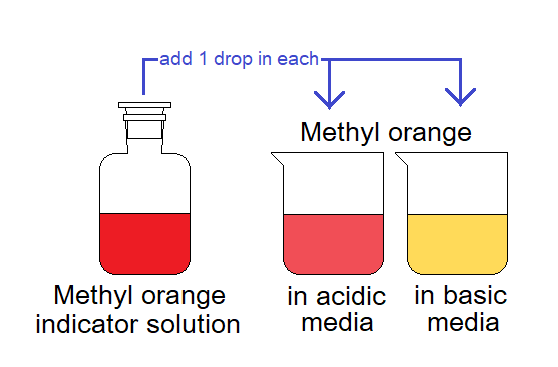
Physical Properties & Chemical Formula:
It is yellow or orange solid and having solubility in water 0.5g/100ml at 20°C.
The chemical formula of methyl orange is C14H14N3NaO3S and having molecular mass 327g/mol. The structural formula is not so simple, containing N,N-Dimethylaniline, azobenzene, and sulfanilic acid, as shown below.
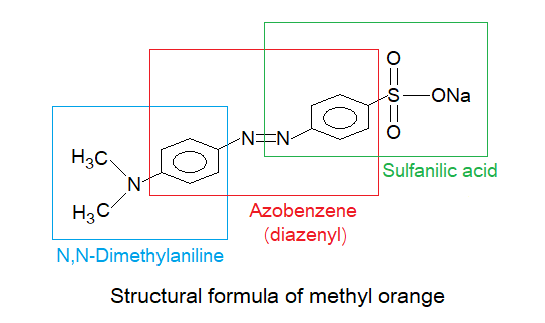
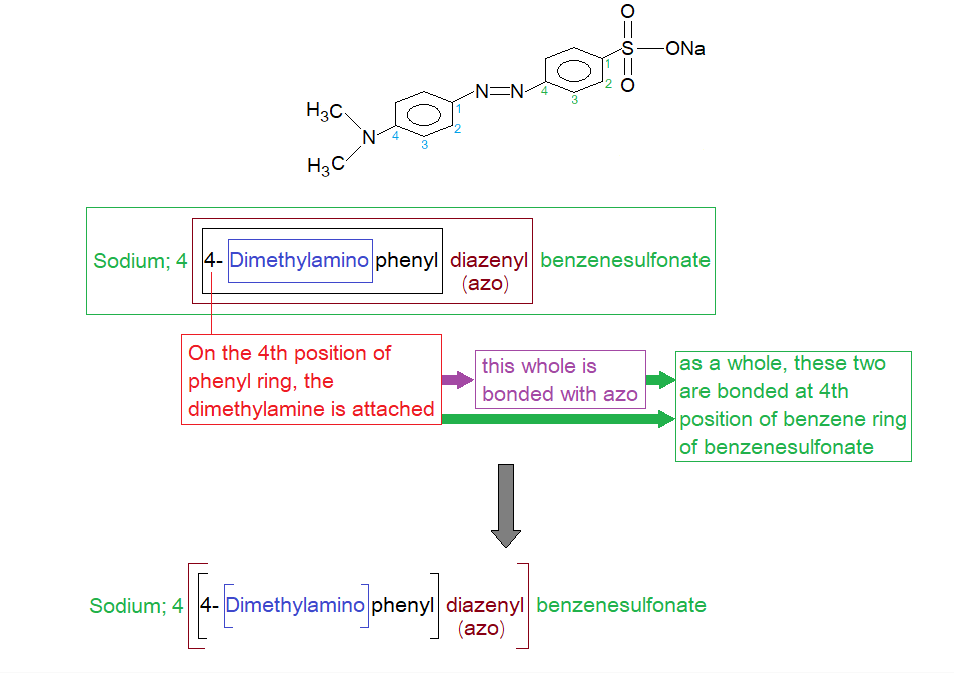
Due to three functional groups, the IUPAC nomenclature comes over a complicated one having the name Sodium; 4-[[4 (dimethylamino)phenyl]azobenzene]sulfonate; azo can be replaced by ‘diazenyl’. Azo etymology is azote (old English), an obsolete word for nitrogen, so azo functional group is diazenyl where two nitrogen atoms are making double bond and are sandwiched between two aryl radicals. On one side of one aromatic ring the sulfonate, while on second ring secondary amine group exists. Thus, the main skeleton is azobenzene (diazenylbenzene: R-N=N-R`), having two functional groups attached on opposite sides.
How, the IUPAC nomenclature of methyl orange comes up? The following diagram can make a conceptual understanding without describing the IUPAC rules.
Solution Preparation in Lab:
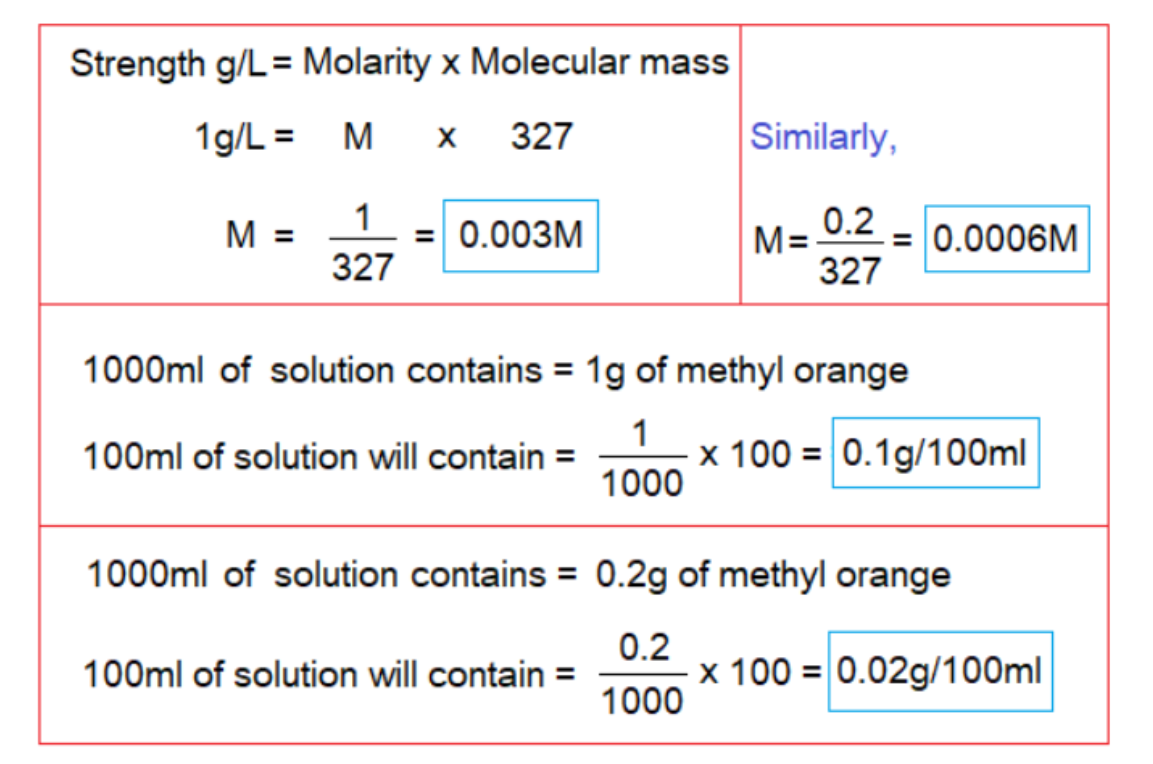
Although, methyl orange is just 0.5g/100ml soluble at 20°C, but, it’s very low molarity is quite enough for its use as indicator. The solution is made in laboratory in distill water with the molarity 0.003 or even 0.0006 molar. For 0.003 molar, its 1g/L (0.1g/100ml); and for 0.0006 molar, 0.2g/L (0.02g/100ml) are used. After dissolving, the solution is filtered and stored in lab. reagent glass bottle. The 0.0006M strength is useful even for a very dilute solutions of acids or bases. It makes a red solution in water.
Methyl Orange & PH:
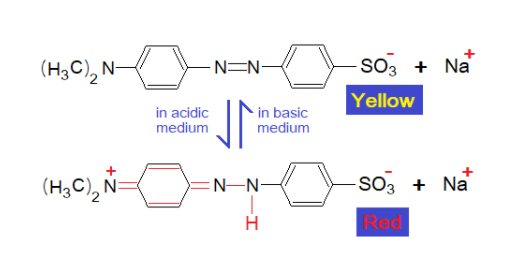
The red colour of the indicator in acidic medium changes at PH 4.4 and turns yellow in basic medium. The structural changes have been shown here. In acidic medium, one of the azo nitrogen atom attains acidic hydrogen and double bond between N, N shifts between azo-N & aromatic ring-C at one side. Resultantly, across that ring, another double bond is created between N of secondary amine and the C of the concerned ring; and as per need the aromaticity of the benzene ring rearranges its delocalized π- π overlapping. In acidic medium, the structure is a zwitter ion, as shown below.
Methyl orange gives its colour change at lower PH; thus, weak base versus strong acid titration is useful here to detect equivalent (stoichiometric) point, where acid and base neutralizes each other. So, Na2CO3 , NaHCO3 are the bases titrated with HCl or H2SO4 in the presence of methyl orange indicator. However, phenolphthalein indicator is used for strong alkalis, such as KOH, NaOH; because, the stoichiometric point is achieved at higher PH 8.2.
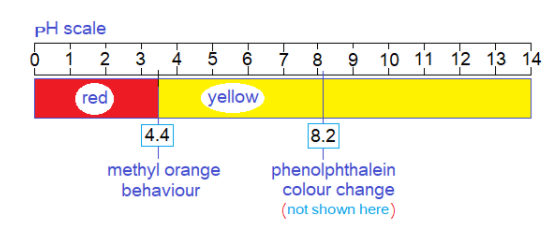
When the PH is above 4.4 in the solution of weak base, the addition of strong acid solution lowers its PH and attains below to 4.4, the red colour then appears. There is a very careful analysis to get the equivalent point as the sharp end-point of volumetric analysis.
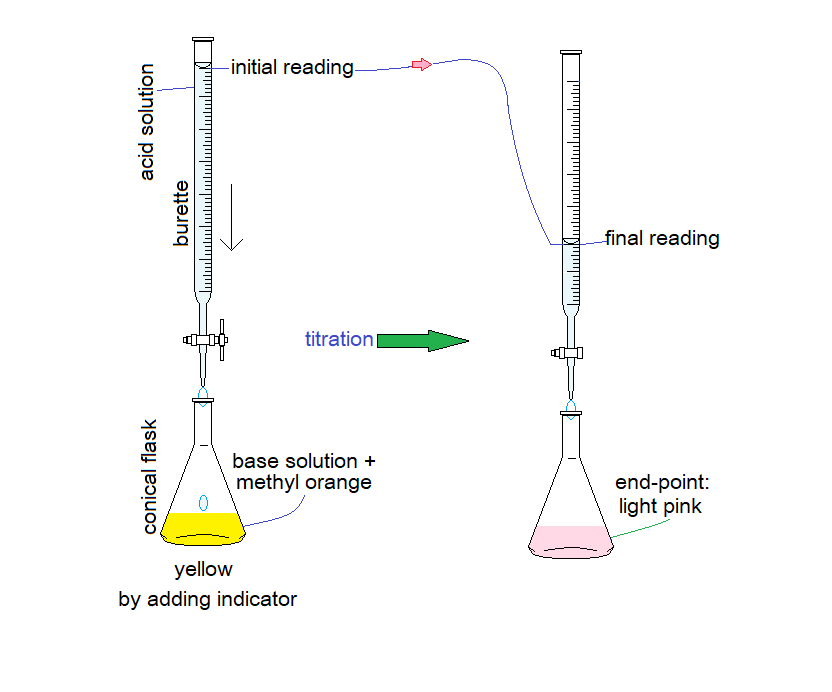
Titration:
The acid or base solution prepared with the molarity 0.1 or 0.05, depending upon their balanced chemical equations, as elaborated below. Base is usually taken in Erlenmeyer (conical or titration) flask; further, 1 or 2 drops of indicator are added. Acid is mostly taken in burette. In case of taking base in burette, the nozzle and stopcock can be blocked; however, an acid can make inner side of the tube clean.
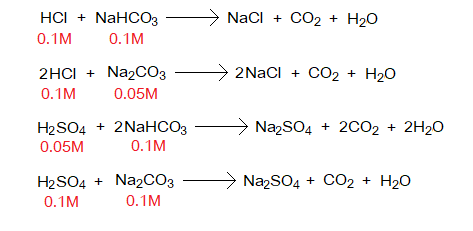
The procedure of titration is simple. The base solution turns yellow by adding methyl orange indicator. From the burette, drop-by-drop acid solution is added into the conical flask with constant whirling the flask by hand. Be careful to get titration stopped just before the end point. Just by dropping one or two more drops of acid solution the end-point should be achieved. This is equivalent point where the conical flask’s solution turns light pink. Note the volume of acid solution used from the burette, by subtracting initial burette reading from the final one; then, take at-least three concordant readings; and calculate their average. Thereafter, all the results are based upon calculations.
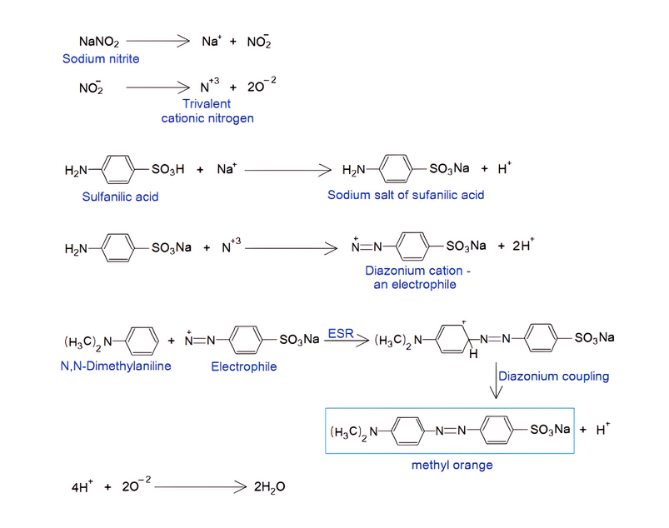
Synthesis of Methyl Orange Compound:
Sodium nitrite releases a trivalent cationic nitrogen and sodium cation. Sulfanilic acid makes its sodium salt with Na+ . The nitrogen of amine of this salt reacts with trivalent cationic nitrogen by replacing 2H+ ions; and makes a N, N double bond with electrophilic properties and called diazonium cation (E+). This E+ attacks at para position of aromatic ring of N, N-Dimethylaniline to play an electrophilic substitution reaction (ESR); and by mechanism called diazonium coupling, the methyl orange compound is formed.

There’s a quality in your writing that transcends the typical bounds of literature. It doesn’t simply convey ideas — it invites the reader to explore them from within, to sit with them in silence and feel their deeper meanings settle. This is the sort of writing that not only informs, but transforms. You’ve managed to make something as ordinary as words into a space where one can feel truly alive.