Dr. Mudassar Altaf, Associate Professor, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Introduction of the structure
- Synthesis
- Mechanism
Introduction:
Beta-amino-carbonyl is a Mannich base that was developed by a German pharmaceutical chemist Carl Ulrich Franz Mannich (1877-1947). The nitrogen atom, in structural formula, is attached with beta-carbon of the compound as shown in the following structure.
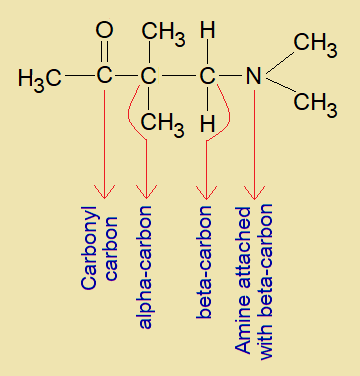
Synthesis:
Mannich reaction is a condensation reaction between aldehyde or ketone and ammonia or primary or secondary amine to form beta-amino-carbonyl compound.
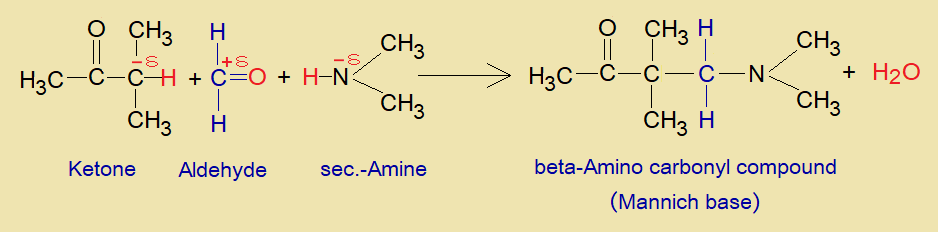
The partial negative alpha-carbon of aldehyde or ketone, the partial positive carbonyl-carbon of another aldehyde or ketone, and the nitrogen of amine or ammonia containing lone pair of electrons link up to make the compound called Mannich base.
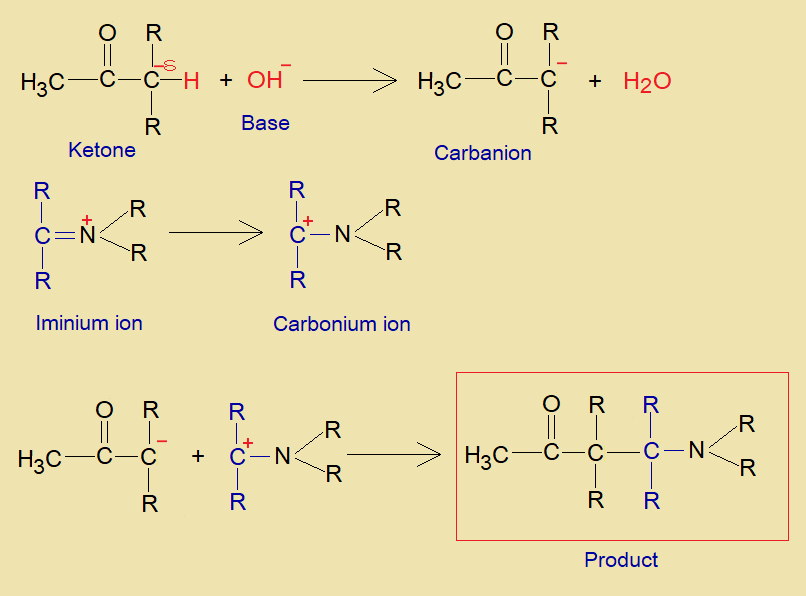
Mechanism:
- The acidic hydrogen makes hydroxyl bond with oxygen of aldehyde or ketone to form carbonium ion (carbo-cation). The amine donates lone pair of nitrogen to this positively charged carbon and ultimately forms iminium ion by dehydration. The iminium ion carries positive charge on nitrogen and an unsaturation with carbon.
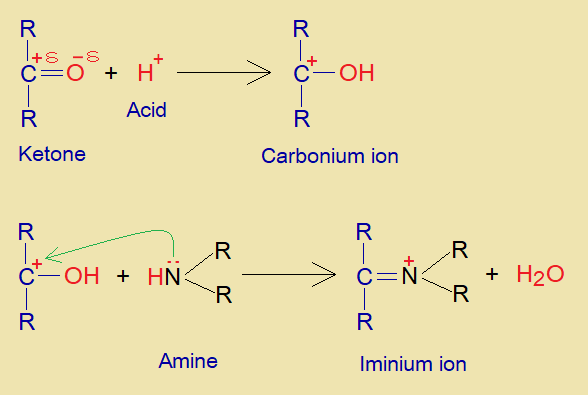
- Another aldehyde or ketone undergoes into deprotonation by a base and makes carbanion that acts as active methylene compound.
- The iminium ion makes carbonium ion that then makes a bond with carbanion as shown below. Ultimately, ß-amino carbonyl compound is formed.