Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Hydrogen-oxygen fuel cell: definition
- Components of the cell
- Working of the cell
- Disadvantages of fuel cells
- Advantages of fuel cells
Hydrogen-oxygen Fuel Cell:
Definition: It can be defined as, “the electrochemical cell that produces electrical energy by using hydrogen gas as fuel and oxygen gas as oxidant; water is the byproduct by this chemical change”.
The fuel cell was invented by a Briton scientist and judge Sir William Robert Grove (1811-1896) in 1838.
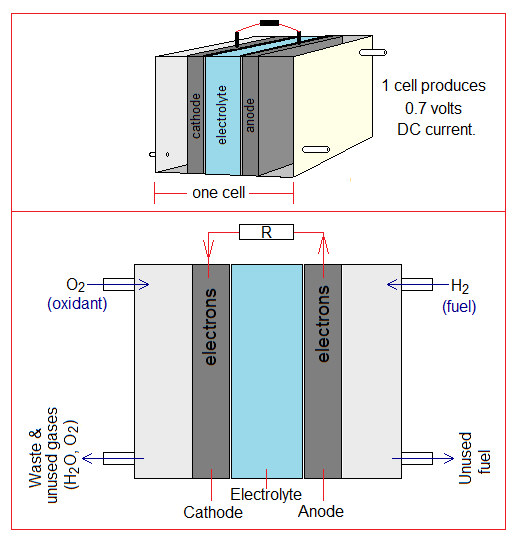
Components of the Cell:
Followings are the major components of the cell:
- Anode: It is the graphite porous carbon rod that contains finally-divided particles of platinum (Pt) metal. The role of Pt is a catalyst.
- Cathode: It is the graphite porous carbon rod that contains finally-divided particles of Pt metal.
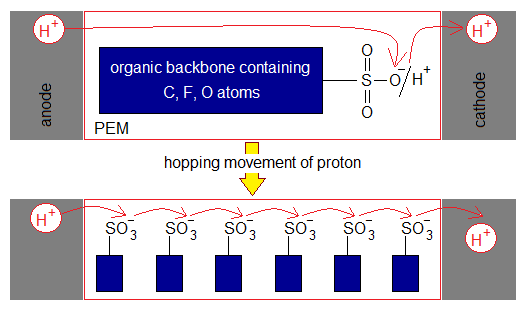
- Electrolyte: (1) The alkaline fuel cell (AFC) contains 30-40% aqueous KOH solution. It is good source of hydroxide ion (OH–). (2) Nafion membrane (developed in 1962) is a first solid synthetic ionic polymer, consisting of ionomers, that is used in proton-exchange membrane fuel cell (PEMFC). It carries H+ ions from anode to cathode. The sulphonate ions (SO3-) loses its acidic hydrogen and the proton from anode makes its loose bonding with its oxide ion. The sulphonates are fixed in membrane, but protons hop (jump) from one to another oxide ion and eventually enter into cathode.
- Nozzles: The cell has four nozzles; two on the anode side and two on cathode side. (1) At the anode side, hydrogen gas as a fuel is allowed to flow into the chamber; and the unused gas is allowed to flush out. (2) At the cathode side, the oxygen gas is allowed to flow in; and the product (water) is discharged.
Working of the Cell:
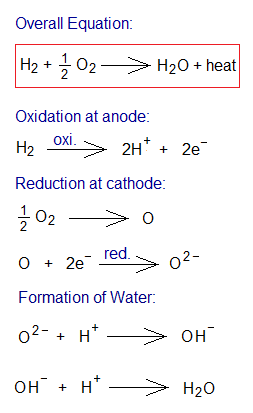
- Oxidation at Anode: At the anode, the hydrogen molecule (H2) dissociates into hydrogen atoms (H) and simultaneously, oxidation takes place to form hydrogen ions (H+). These electrons move towards cathode via external wire.
- Role of Electrolyte: The electrolyte is a mean of transporting H+ ions towards cathode.
- Reduction at Cathode: At the cathode, the oxygen molecule (O2) dissociates into oxygen atoms (O) and then their reduction occurs to form oxide ions. Following that, these ions form hydroxide ions (OH–) and thereupon form water vapours with protons (H+). The change in enthalpy is negative due to exothermic reaction (∆H=-286 kilojoules per mole production of water).
Disadvantages of Fuel Cells:
- Although, cars, buses, motorbikes, aero-planes, boats have been tested successfully and sold out, but commercially the availability of hydrogen gas as a fuel is an issue. Its production on a large scale, storage, transportation are challenges. Further, higher fueling cost cannot make it popular as a fuel for vehicles. On the other hand, by comparison the transportation and storage of gasoline fuel is much easier. Although, hydrogen fuel filling stations were installed in Germany, Japan and other countries, but mostly not working well, circumstances could not be settled in their favour.
- The chemical reaction between hydrogen and oxygen gases is highly exothermic if by chance gases mix together and ignited by a spark or flame, then the reaction would be violent and explosive.
- The hydrogen oxygen cells need storage tanks for hydrogen gas, but can use oxygen from air. Unless, there is no hydrogen storage tanks as external storage system, the cells cannot work. Unlike batteries, the fuel cells don’t store energy; and working is based upon the supply of H2. As compared to Liquid Petroleum Gas (LPG), and Compressed Natural Gas (CNG) that are stored at very high pressure in vehicles’ cylinders. Further, as compared to CNG, the H2 needs its storage almost at the double pressure in cylinders. Thus, cylinders with excessive thick sheets with lower gauge number is required; making them heavier in weight.
- A single cell produces 0.7 volts. For cars, 17 cells are required to generate 11.9 volts for 12 volts running cars. And for buses and heavy vehicles, 24 volts are required; so, 34 cells battery will be required to build up.
Advantages of Fuel Cells:
- Because, the end product (waste) is water, so, no harmful emission is obtained as compared to petroleum where oxides of carbon, sulphur, and nitrogen are produced that cause pollution in air. So, it is environment friendly.
- No noise pollution is produced.
- The efficiency of the fuel cell is 40% and above depending upon the type of fuel cell used.
- Energy power stations can be installed for generation of electricity in the town.