Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Diamond: a macromolecule
- Cutting strength of a diamond
- Graphite: a macromolecule
- Uses of graphite
- Sand: a macromolecule
- Sand & diamond: similarities in properties
Diamond : A macromolecule
The diamond is a giant (big) covalent molecule having only carbon atoms; also categorized as macromolecule. Each carbon atom needs four electrons more to complete its outermost shell by pairing up; and they do so by mutual sharing.
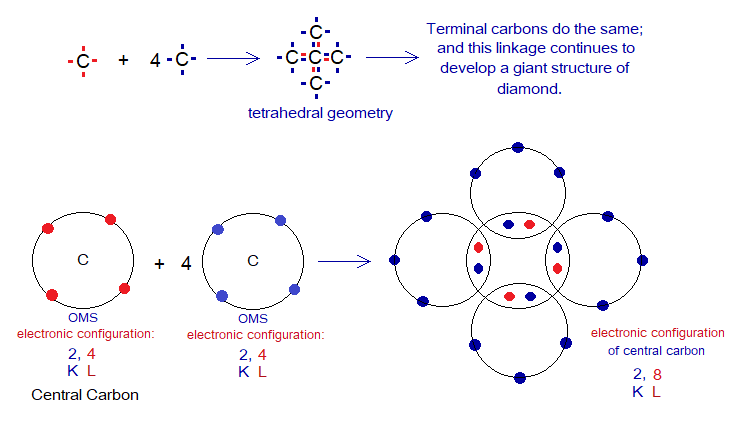
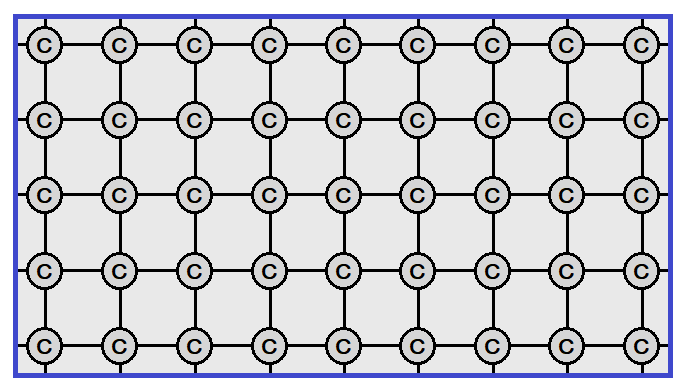
The carbon atom has the geometry of tetrahedron (four sided). In three-dimensional space, it has four orientations and each contains unpaired (1 = single) electron. Thus, each carbon atom links with four carbons for pairing up of electrons by mutual sharing and the linkage continues to develop a giant structure, a network of carbon atoms called ‘diamond’. Each carbon atom is a central atom for other four to which it links.
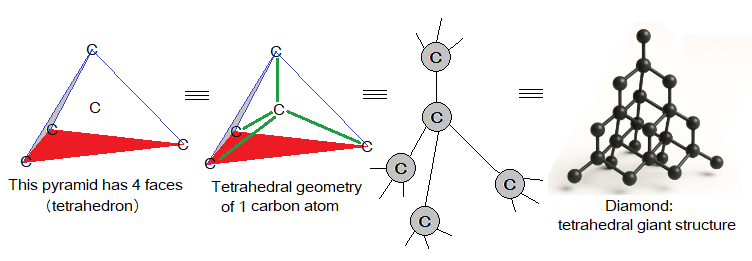
For understanding, a two-dimensional network view elaborates that each carbon atom is interlocked by four other carbon atoms. So, a tetrahedral geometry is a set of five carbon atoms, but having four faces (sides).
Cutting Strength of a Diamond:
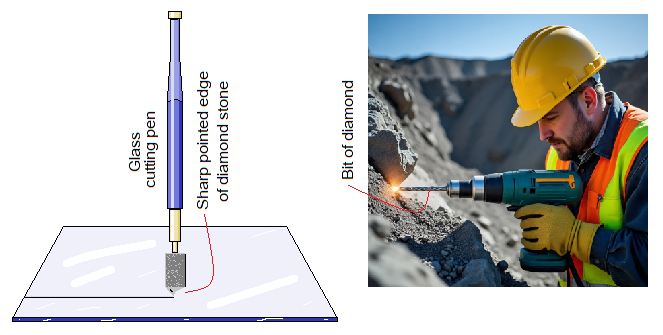
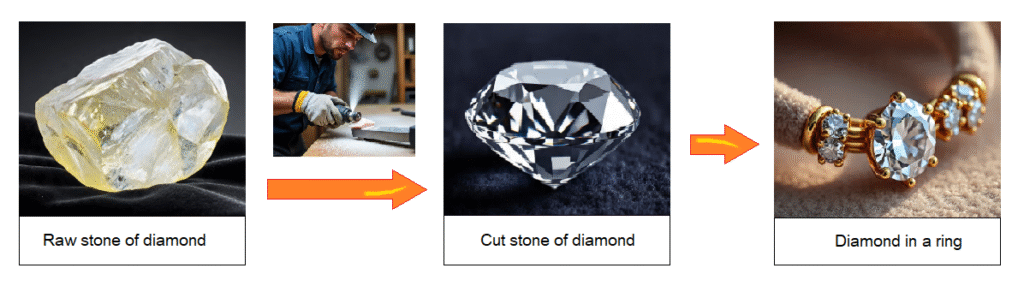
The giant structure of pure carbon atoms makes the diamond extraordinary a hard substance. The etymology of diamond is Greek from the word ‘adamas’ having the meanings of ‘unbreakable’. Due to this property, its raw stone is used to cut the glass. Moreover, drill bits are manufactured with diamond stones for drilling into rocks.
Raw stone of the diamond does not shine as the diamond is famous for its shining. It is cut carefully to proper shape in factories so that can glow by refracting light called a gem.
Graphite: A Macromolecule
Pure carbon has two allotropic forms, diamond and graphite. By definition, allotropy can be defined as, the existence of an element in more than one structural form and having different physical properties but similar with respect to chemical composition”.
Like diamond, graphite is another allotropic form of carbons’ atoms only. But structurally it is different to diamond. Its structure is composed of layers. These layers are actually hexagonal arrangements of carbon-atoms. The hexagonal geometry is not a single one but a repeating pattern in the layer and are interlocked, as shown in the following diagram. Carbon atoms are covalently bonded and exist at each corner of the geometry.
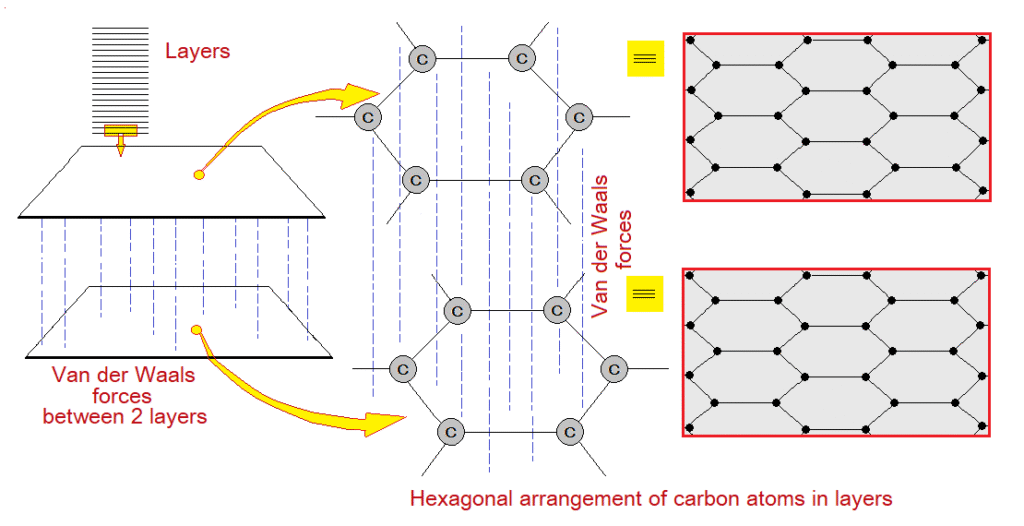
The layers are sliding over each other; having Van der Waals forces, where delocalized electrons move, making the graphite semi-conductor, although not as the metals. On the other hand, diamond does not have free electrons, and thus it is totally insulator.
Uses of Graphite:
- It is used in the core of pencil with the percentage of 35 to 80 in the clay with a little wax as binder. More the percentage of graphite, more will be the core hard, depending upon the hardness and softness of the core adjustment. So, everyone is familiar with its use in writing.
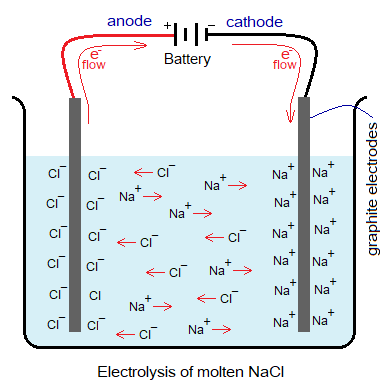
- It is used as electrode rods for electrolysis, connected with the terminals of the battery using wires.
- Graphite is used as lubricant in dry powdered form (less than 10 microns particle size), mixed with water, mixed with oil as grease, or mixed with a suitable solvent to make an aerosol spray. As lubricant, it is used in automobiles, locks, brake systems etc. It can resist high temperature, so used where stability of lubricant at high temperature is required.

Sand: A Macromolecule
Etymology: There are different names of sand, like silica dioxide, silica, siliceous sand, silica sand, quartz. It is believed that Quartz has its German origin from the word ‘Quarz’, but not sure. The meaning of the Quarz is ‘hard’ i.e., in the sense ‘a hard mineral’. The word ‘Sand’ is also a German word having its meaning ‘a granular substance’. There are various sizes of sand particles; commonly it is 0.03mm (3/100) to 1mm in its radius. There are various colours of sand, white to grey depending upon the impurities present.
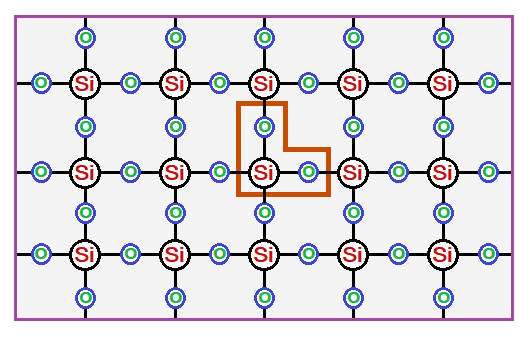
Formula: The formula of sand is SiO2; so chemically, it is silica dioxide; an oxide of silicon. SiO2 is a ‘simple whole number ratio’ of Si2O4; dividing by 2. Because, oxygen is covalently shared with 2 Si atoms; while Si is covalently shared with 4 oxygen atoms. The Si is tetrahedral in its geometry and makes a polymer lattice with oxygen atoms, as shown in the following diagrams. The repeating unit is SiO2 as shown in tan box below.
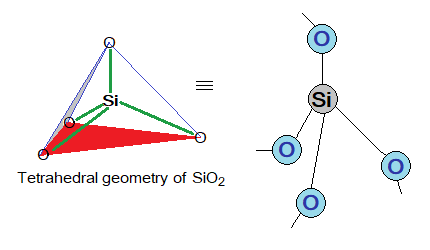
Sand & Diamond: Similarities in Properties
There are many similarities in properties, particularly related to structure, between diamond and sand, as under:
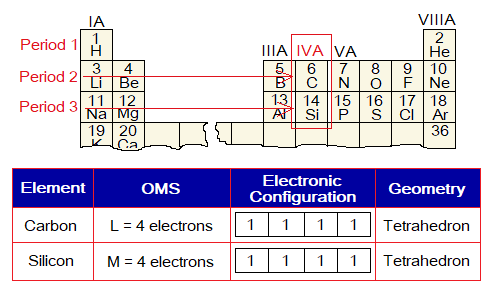
1. Carbon and silicon both have similar electronic configuration of their outermost shells (OMSs). Because, both belongs to IVA group (carbon family) of the periodic table. However, OMS of C is L; while that of Si is M; carbon belongs to period 2, while Si belongs to period 3 of periodic table. Both have four unpaired electrons in their OMSs and form tetrahedral geometry. Due to valency 4, the silicon dioxide is also written as silicon (IV) oxide. Further, both C & Si form 4 single covalent bonds in their compounds, diamond and quartz respectively.
2. Both are solids having strong covalent network structure making them a giant structure. Thus, their melting points are also high; m.pt. of quartz is 1710°C and that of diamond is 3550°C.
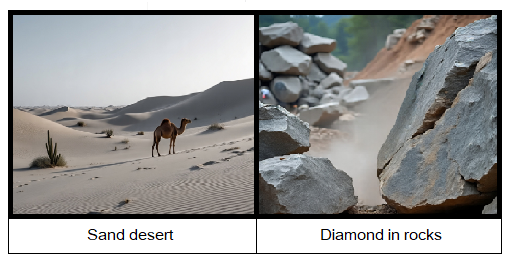
3. Sand naturally found in deserts in huge amount, making sand deserts. Rub’ al Khali is a largest desert in Arabian Peninsula covering 650000 km2 area including Kingdom of Saudi Arabia, United Arab Emirates, Oman & Yemen. Second one is Sahara Desert in North Africa. These are very hot deserts and their temperature goes up to 55°C even above to that. On the other hand, diamond exists in rocks.
4. Both are insoluble in water due to giant covalent structures.
5. Both are insulators. Because, there is no free electrons or ions exist in their structure.
6. Sand is commonly used in construction of buildings; while diamond is used in jewelries as gemstone because of glowing and sparkling by refraction and internal reflection of light. Further due to hardness, diamond is also used in cutting tools for glass and rocks.



My brother suggested I might like this website. He was entirely right. This post truly made my day. You can not imagine just how much time I had spent for this info! Thanks!