Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Symbols of elements
- Compounds and their formulae
- Formula of covalent compounds and their types
- Valencies and their types
- Formula of ionic compounds
- Balancing of charges
- How to write a chemical equation?
- Mole
- Stoichiometry and moles, definition and features
- Ionic equations
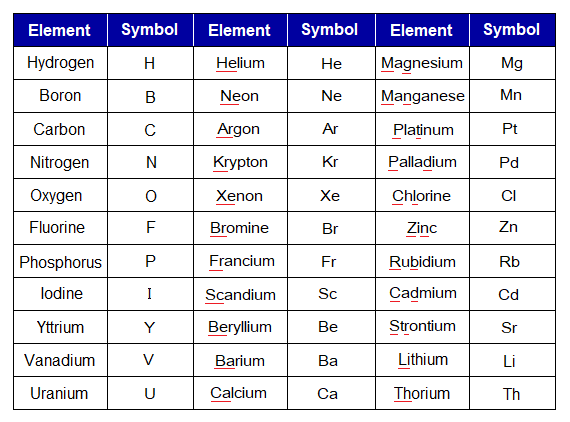
Symbols of Elements
Definition: “The short-form of writing the elements is called symbols of elements”. There are different but fixed forms to express elements by the symbols; these are by using:
- First letter but capital of their English names; for example, N for nitrogen.
- First letter but capital of their English names with second or third letter but small; for example, Ne for neon; Mg for magnesium.
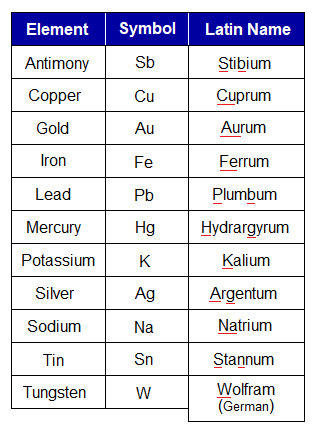
- First letter but capital of their Latin/Greek names; for example, K for potassium due to its name kalium.
- First letter but capital of their Latin names with second or any other letter but small; for example, Na for sodium due to its name natrium.
Following tables help to know the symbols of various elements. Go through, how these were formed by using their names, either English or Latin/Greek.
Compounds and Their Formulae:
By definition, “the compounds are pure substances that are formed by chemical combination between atoms either similar or dissimilar”.
The term ‘formula’ is singular; have its plural forms ‘formulae or formulas’. Its origin is Latin from the word ‘forma’ meaning ‘mould or shape’. It can be defined as, “the way of expressing the compounds in short form” is called formula. The chemical combination is either covalent bonding or ionic.
1. Formula of Covalent Compounds:
The formula of a covalent compound represents the exact number and types of the atoms chemically bonded together in a fixed ratio. For example, H2O is the formula of water, describes two hydrogen atoms and one oxygen atom that make the molecule. This formula of water has fixed ratio and can never be changed. If changed then that will be another compound, for example, H2O2 is not water but hydrogen peroxide. Similarly, CO2 is carbon dioxide; while CO is carbon monoxide. These can be classified into following types:
- Diatomic molecules: Formed by the covalent bonding between two atoms; for example, H2, O2, N2, CO, HCl, hydrogen gas, oxygen gas, nitrogen gas, carbon monoxide gas, hydrochloric acid etc. respectively.
- Triatomic molecules: Formed by the covalent bonding between three atoms; for example, H2O, CO2, O3, SO2, NO2, N2O, water, carbon dioxide gas, ozone, sulfur dioxide gas, nitrogen dioxide gas, dinitrogen monoxide gas etc. respectively.
- Tetratomic molecules: Formed by the covalent bonding between four atoms; for example, H2O2, NH3, C2H2, hydrogen peroxide, ammonia gas, ethyne (acetylene) gas etc. respectively.
- Pentatomic molecules: Formed by the covalent bonding between five atoms; for example, CH4, HCOOH, HNO3, methane gas, formic acid, nitric acid etc. respectively.
- Polyatomic molecules: Poly means many; so, these are formed by the covalent bonding between atoms more than five; for example, C6H12O6, C12H22O11, CH3OH, C2H5OH, C6H6, C3H6O, H2SO4, CH3COOH, glucose, sucrose (table sugar), methanol, ethanol, benzene, acetone, sulfuric acid, acetic acid etc. respectively.
- Macromolecules: Mostly, these are very large covalent molecules formed even by thousands of atoms. The examples of such types of molecules are polysaccharides, proteins, fats and oils (lipids), polymers, nucleic acids. Polysaccharides and polymers have monomers as smaller repeating units. Starch is one of the examples of polysaccharide and having its formula (C6H10O5)n. The ‘n’ represents the number of monomers in the molecule; might be 300 to 3000 units (monomers) of C6H10O5 bonded together by covalent linkage. Polythene is a polymer having formula: ̶(CH2-CH2)̶ n. Its monomer is ethene molecules making a long chain of units ̶(CH2-CH2)̶ .
Valencies:
“The combining capacity of an atom is called its valency”. Followings are the types of valencies:
- Monovalent atoms: The atoms having 1 valency, i.e., they can make one bond only. For examples, H, F, Cl, Br, I.
- Divalent atoms: The atoms having 2 valency, i.e., they can make two bonds. For examples, O, S (as in H2S).
- Trivalent atoms: The atoms having 3 valency, i.e., they can make three bonds. For examples, N, P (as in PCl3: phosphorous trichloride)
- Tetravalent atoms: The atoms having 4 valency, i.e., they can make four bonds. For example, C, S (as in SO2 & H2SO3).
- Pentavalent atoms: The atoms having 5 valency, i.e., they can make five bonds. For example, P (as in H3PO4: phosphoric acid; H3PO3: phosphorous acid; PCl5: phosphorous pentachloride).
- Hexavalent atoms: The atoms having 6 valency, i.e., they can make six bonds. For example, S (as in SO3 & H2SO4).
- Variable valency state: Some atoms show variable valencies, for example, S shows 2 (in H2S), 4 (in SO2, H2SO3), and 6 (in SO3, H2SO4); following structural formulae can elaborate more clearly.
Remember that maximum three covalent bonds can be formed between two nuclei; more than three is impossible. None of the bond is formed tetra, penta, hexa, hepta etc. between two nuclei.
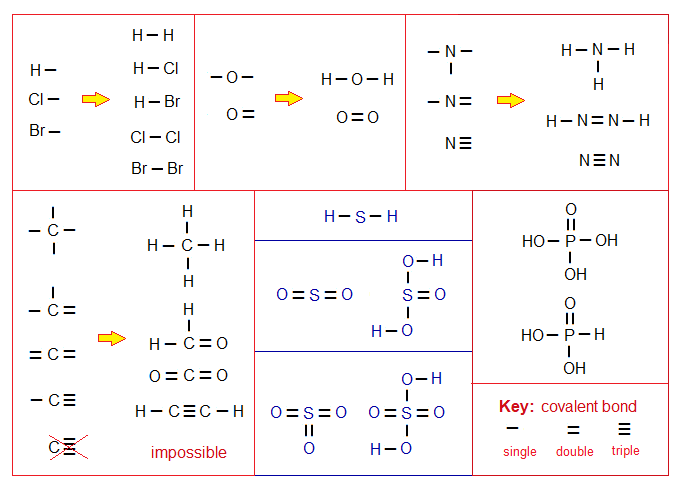
Exercise 1:
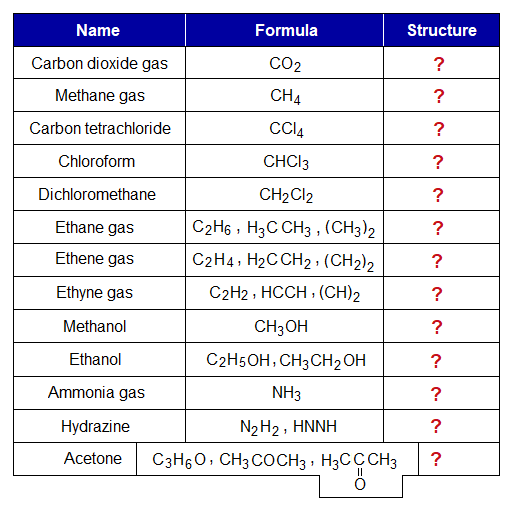
Draw the structural formulas of the following compounds just for the practice, because, many of them are known. And learn balancing of valencies when atoms make covalent molecules. Note: Structural formulas are drawn by using lines of covalent bonds either single, double or triple, as drawn above.
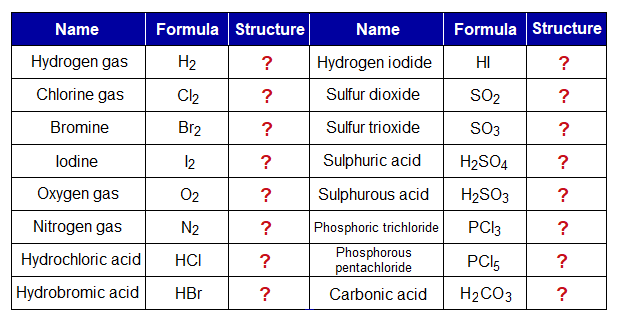
Exercise 2:
Draw the structural formulas of the following compounds given in the table; and learn balancing of valencies when atoms make covalent molecules.
2. Formula of Ionic Compounds:
The formula of an ionic compound represents the types of the ions (cations and anions) chemically bonded together in the form of cluster of ions by electrostatic forces of attractions and is written in a simple whole number fixed ratio. For example,
- Sodium chloride has its chemical formula NaCl. It is a cluster of sodium as well as chloride ions but cations and anions make a fixed ratio of 1:1 between Na+ and Cl– ions.
- Calcium chloride has its chemical formula CaCl2. It is a cluster of calcium as well as chloride ions but cations and anions make a fixed ratio of 1:2 between Ca2+ and Cl– ions respectively.
- Sodium oxide has its chemical formula Na2O. It is a cluster of sodium as well as oxide ions but cations and anions make a fixed ratio of 2:1 between Na+ and O2- respectively.
Mostly, cations (+ive ions) are written on the left side; while anions (-ive ions) on the right side, as in above examples. But it is not true in all cases, like lead acetate (CH3COO)2Pb. The Pb2+ is written on right side; while acetate ion CH3COO– on left side.
Balancing of charges:
The formula of an ionic compound depends upon how the charges of cation(s) and anion(s) are balanced to make the molecule neutral. Few examples are given as under:
- 1 Na+ needs 1 Cl– to make a neutral NaCl molecule.
- 1 Ca2+ needs 2 Cl– to make a neutral CaCl2 molecule.
- 1 O2- needs 2 Na+ to make a neutral Na2O molecule.
- 1 Al3+ needs 3 Br– ions to make a neutral AlBr3 molecule.
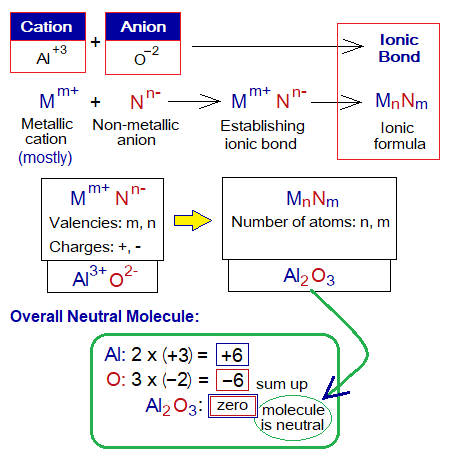
The molecule formed is overall neutral. How it becomes neutral? Let’s study an example of Al2O3 (aluminium oxide). Aluminium is Al3+, and oxygen is O2-. The valencies are used for cross multiplications; the answers tell the number of atoms required; as 2(Al3+) and 3(O2-) = Al3+ + Al3+ = 6+; and O2- + O2- + O2- = 6-. In other words, if 3 is multiplied by 2 then it will be 6 and vice versa. So, two aluminium ions and three oxide ions will balance each other. So, 3 of aluminium (valency only) will come under O for the number of oxygen atoms. Likewise, 2 of oxygen will come under Al for the number of oxygen atoms.
Exercise 3:
Here is a list of cations and anions; make ionic molecules as many as you can do. Cations: Li+, Na+, K+, H+, NH4+, Mg2+, Ca2+, Ag+, Fe2+, Cu2+, Al3+ lithium cation, sodium cation, potassium cation, hydrogen cation (proton), ammonium ion, magnesium cation, calcium cation, silver cation, iron cation, copper cation, aluminium cation respectively. Anions: Cl–, Br–, I–, H–, CH3COO–, O2-, N3-, C4- chloride ion, bromide ion, iodide ion, hydride ion, acetate ion, oxide ion, nitride ion, carbide ion respectively. Remember, cations are mostly metals except NH4+ (ammonium ion, a non-metallic ion); while anions are non-metals.
Thought Provoking: Iridium (Ir, atomic number 77) has highest cationic valency recorded, i.e., +9. However, the recorded highest anionic valency is -4 and one of the example is carbon (carbide C4-).
How to Write a Chemical Equation:
Chemistry deals with chemical reactions and there is a need to express equations and its features in a short form. The chemical reaction has two major components on the page, these are:
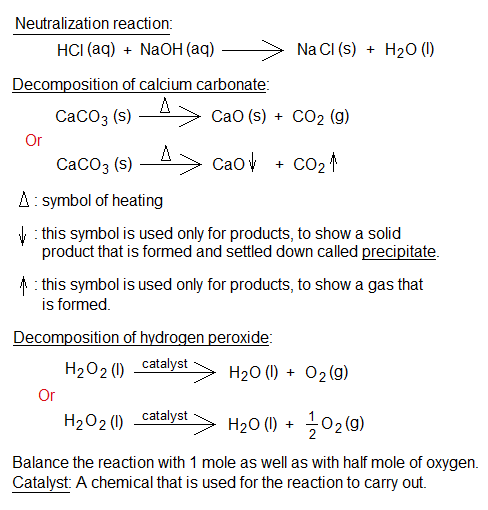
- Reactants: The substances that react chemically together are called reactants. The reactant might be a single substance if it is going to change its chemical composition. For example, thermal decomposition of CaCO3 into CaO and CO2.
- Products: The substances that are formed by means of chemical reaction between reactants. In the above example, CaO and CO2 are products; while CaCO3 is a reactant.
Followings are the steps to write a chemical equation.
- First of all, the reaction is expressed in word form.
- Then, the reactants and products are expressed in their symbolic forms (ions, atoms, molecules). Reactants are written on left side and products are on right side, having an arrow in-between. The arrow head is directed towards products.
- The states of the substances are expressed in brackets by (s), (l), (g), (aq); s stands for solid state, l for liquid, g for gas and aq for aqueous solution.
- Next step is balancing of equation. In a balanced equation, masses in grams on both sides of arrow are equal.
Examples:
Word Form: When an acid reacts with a base, salt and water are produced. Suppose, sulfuric acid (H2SO4) when reacts with sodium hydroxide base (NaOH), the salt sodium sulphate [Na2(SO4)] and water are produced.
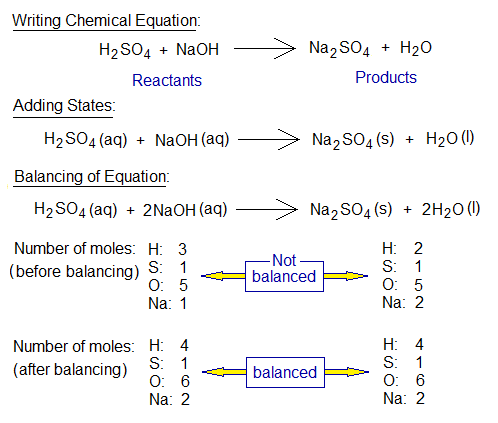
Standard Form: The balanced chemical equation is normal one and is a standard formation. So, the above chemical reaction uses 1 mole aqueous solution of H2SO4 and 2 moles aqueous solution of NaOH to yield 1 mole solid salt of Na2SO4 and 2 moles of H2O; is all about its standard form (the normal one).
Exercise 4:
Balance the following chemical equations.
Mole:
By definition amole is “atomic or molecular mass of a substance, either atom, ion or molecule when expressed in grams for its quantitative measurement”.
This term was coined by a German Nobel Laureate chemist, Wilhelm Ostwald (1853-1932) in 1894. The origin of the term is Latin ‘moles’ meaning ‘large amount, large number of particles, a heap’.
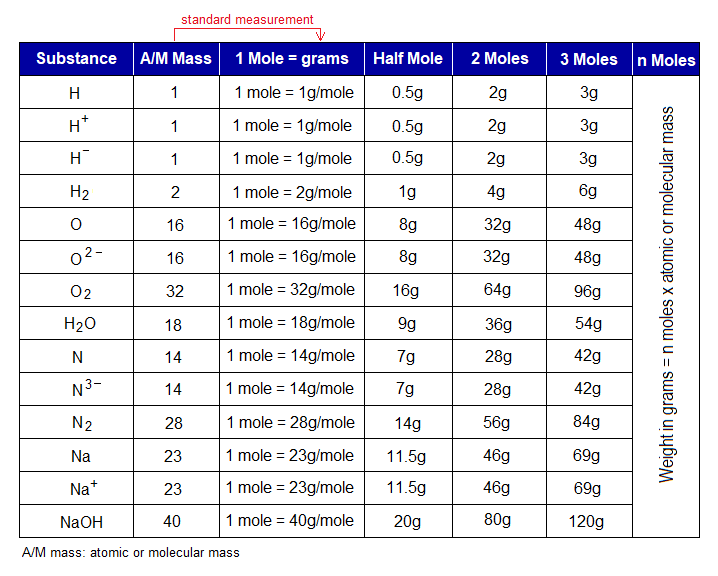
One mole is a standard measurement; and equals to atomic or molecular mass. The number of moles is multiplied with atomic or molecular masses to get desired amount in grams of the substance. Following table shows atomic or molecular masses of few selected examples; then mass in grams for 1 mole (standard measurement), ½ mole, 2 moles, 3 moles and ultimately n moles have been expressed.
How much the weight of the substance is required in grams, can be calculated and measured by using the formula given in the following table.
Stoichiometry and Moles:
A German chemist Jeremias Benjamin Richter (1762-1807) coined the term stoichiometry (pronunciation: stoy-ke-o-metry). The etymology is Greek by the combination of two words, ‘stoicheion’ and ‘metron’, meaning ‘element’ and ‘measurement’ respectively. By definition, stoichiometry is a term in chemistry that “deals with the quantitative relationship between reactants and products in a chemical reaction”.
Features of Stoichiometry:
- The chemical equation should be written complete.
- The equation must be balanced.
- It is not necessary that number of moles should be balanced on both sides of arrow.
- It is an essential feature that sum of masses of reactants and products should be equal.
Go through the following two examples.
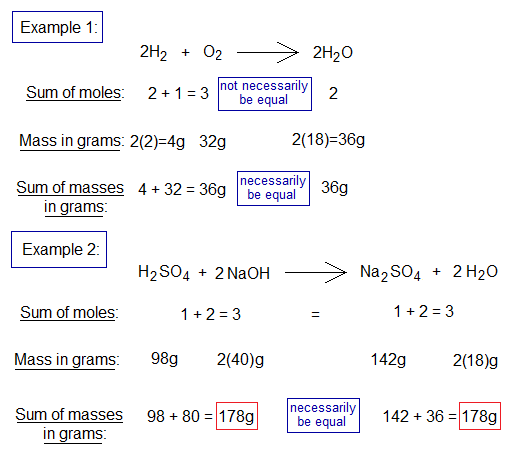
The above chemical reaction, in its standard form, uses 1 mole (98 grams) of H2SO4 and 2 moles (80 grams) of NaOH to yield 1 mole (142 grams) of Na2SO4 and 2 moles (36 grams) of H2O. As we know that molecular mass when taken in grams of the substance is its 1 mole. So, it is mandatory to know, how molecular masses (weights) are measured? It is the sum of all the atomic masses as described below.
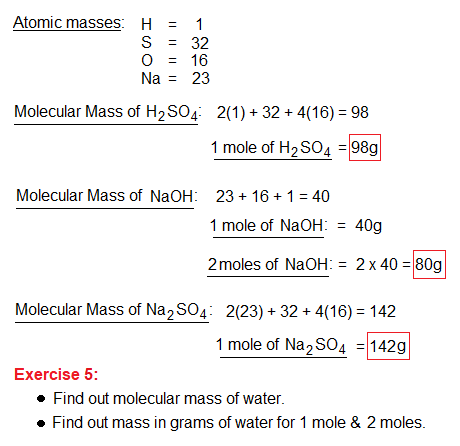
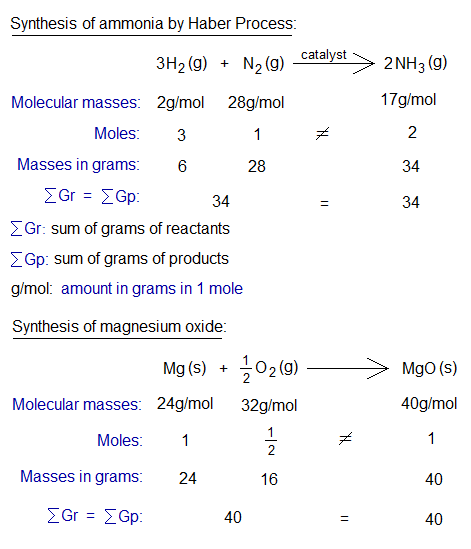
Few other examples are given below.
The concept of stoichiometry is applied in the sense that prior to perform the chemical reaction, it is theoretically calculated. For example, for the yield of 100g of zinc sulphate, following calculations are required for a particular chemical reaction selected for the purpose.
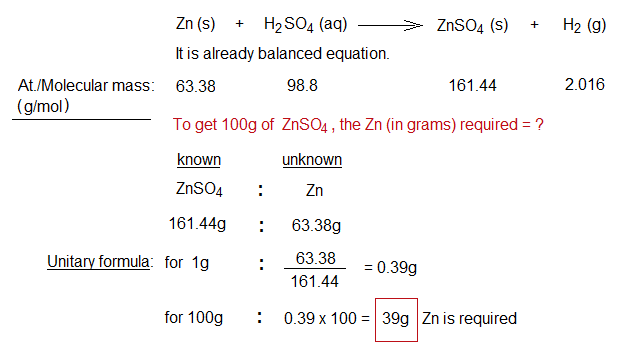
The value 39 grams is not quite enough; and further calculations are also required as explained below.
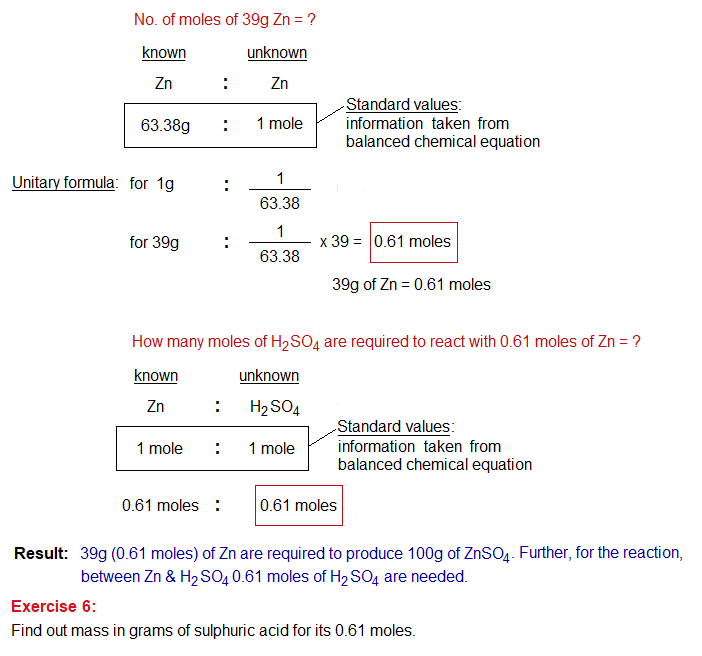
Ionic Equations:
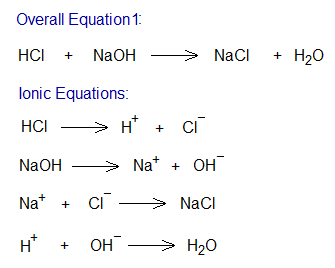
It can be defined as, “the chemical equation written in the form of ions, either reactants or products or both”.
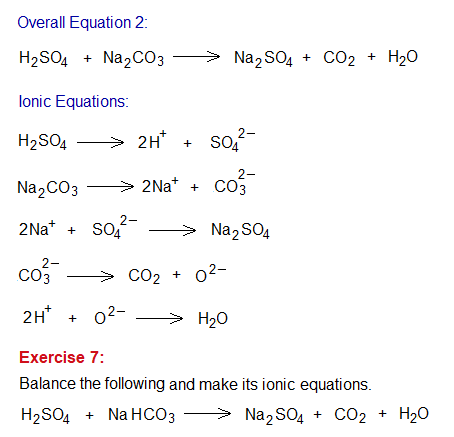
Study the following example of neutralization reaction between an alkali (NaOH) and an acid (HCl). During a chemical change, old bonds break and new ones are formed. The overall equation can be written step-by-step by relevant ionic equations, as shown below.



Good – I should certainly pronounce, impressed with your web site. I had no trouble navigating through all the tabs and related info ended up being truly simple to do to access. I recently found what I hoped for before you know it in the least. Quite unusual. Is likely to appreciate it for those who add forums or anything, web site theme . a tones way for your customer to communicate. Nice task.