Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Definition and etymology of empirical formula
- Empirical formula of ionic compounds
- Empirical formula of covalent compounds
- Finding empirical formula from the data of combustion analysis
- Finding molecular formula from empirical formula
Empirical Formula:
By definition, “the simple whole number ratio between atoms or ions in a molecule” is called an empirical formula. The whole number means, 1, 2, 3, 4 ……; while the fractions like 0.25, 0.5, or 0.75 …. are not used.
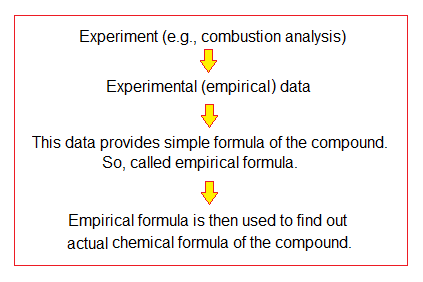
The origin of the term is Greek from the word ‘empeiria’ meaning ‘experience, observation, experiment’. Thus, empirical means ‘based upon the data collected by means of empeiria’. The empirical formula is derived by the experimental data in chemistry laboratory. This is initial and basic step of determining molecular formula. Empirical formula is then used to find out molecular formula.
Empirical Formula of Ionic Compounds:
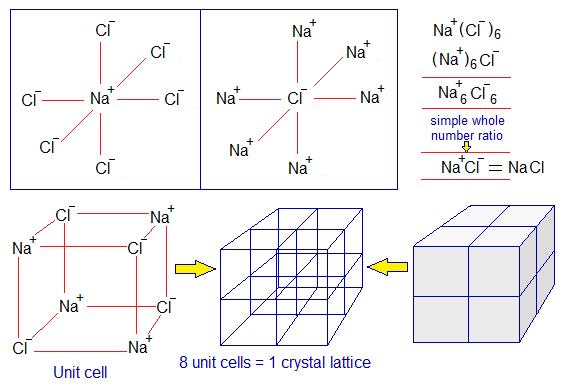
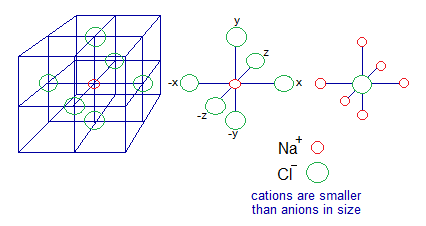
The ionic compounds are cluster of cations and anions. For example, in NaCl crystal, each Na+ is surrounded by 6 Cl– ions; and each Cl– is surrounded by 6 Na+ ions. So, the simple whole number ratio will be 1:1 for Na+ and Cl–. Likewise, for all other examples of ionic compounds. NaCl crystal is cubic and consisted of 8-cubic unit cells. Cations and anions are arranged in alternative positions along the length, width or height (±x, ±y, ±z axis). So, this arrangement gives a shape in which each Na+ is surrounded by 6 Cl– ions and vice versa. If a simple whole number ratio is calculated then 1:1 is obtained.
There are few ionic compounds written with actual number of atoms rather than simple whole number ratio, like sodium peroxide (Na2O2). Because, it contains two ions: Na+ and O22- [peroxide ion: (O-O)2-].
Empirical Formula of Covalent Compounds:
Unlike ionic compounds, it is much easier to understand empirical formula of covalent molecules. It is a mathematical calculation of following steps:
- First of all, write molecular formula, for example, C6H12O6 of glucose.
- Divide subscripts (6, 12, 6) by a greatest common divisor (GCD). It is 6 in this case. So, it will be 6/6=1, 12/6=2, 6/6=1.
- Now, use the quotients (the answers of divisions) as subscripts below symbols of atoms in the same sequence. So, the empirical formula will be CH2O for glucose.
Few examples are given in the following table; each molecule has its own GCD.
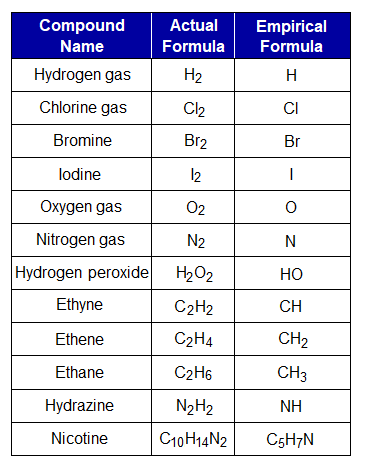
There are numerous molecules having empirical and actual molecular formula same. Go through the examples, you will find no difference between the two. Because, there is no GCD among such examples; H2O, NH3, H2SO3, H2SO4, SO2, SO3, HCl, HBr, HI, CH4O (methanol), C2H6O (ethanol) etc.
How to Identify Molecular Formula Empirically?
It has two steps. First to identify empirical formula, then finding molecular formula.
1. Finding Empirical Formula:
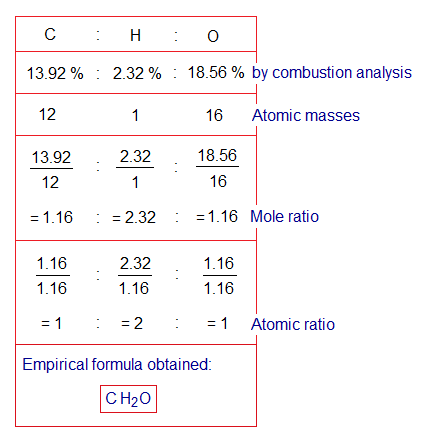
By combustion analysis, the percentages of the elements of the organic compound obtained as C=13.92%; H=2.32%; O=18.56%. The experimental work ‘combustion analysis’ and ‘calculations of percentages’ have not been interpreted here due to curriculum limitations. However, simply, it must be known that the organic compounds (O.C) produce CO2 and H2O upon burning with O2 gas; from the known amount of O.C. the amounts of CO2 and H2O produced are measured in grams and then the formula is applied to calculate the percentages of the elements. However, further steps of mathematical calculations have been described as under:
- Mole ratio: Divide the percentages by relative atomic masses of the elements to get the quotients as mole ratio between elements.
- Atomic ratio: Divide each mole ratio with least value among above quotients to get new quotients as atomic ratio, e.g., 1.16 in this case.
- Empirical formula: Use atomic ratios to formulate empirical formula. Following example formulates CH2O.
2. Finding Molecular Formula:
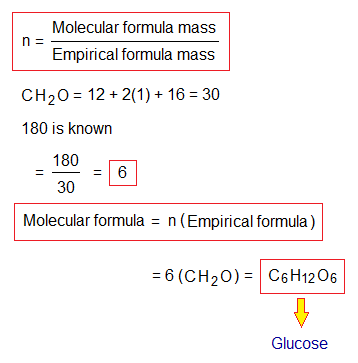
For finding molecular formula from empirical formula, the mass of molecular formula (mm) must be known (given); while, the empirical formula mass (me) is calculated. Thereafter, the value of ‘n’ is determined by means of a formula given below. The ‘n’ is a ratio between mm and me. The mm is nominator (as dividend) and me is denominator (as divisor). So, the ‘n’ value obtained is multiplied with subscripts of the empirical formula to find out the molecular formula. Go through the following example:
Exercise 1:
- Combustion analysis of an organic compound shows that it contains 85.63% carbon and 14.37% hydrogen. Find out empirical formula.
- From the empirical formula determined in first step of this exercise, find out molecular formula. The molecular formula mass is 84.



I coᥙldn’t resist commenting. Exceptionally well written!
Also visit my blog post Dewa77