Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Experimental verification 1: diffusion between hydrogen gas & air
- Experimental verification 2: diffusion between carbondioxide gas & air
- Experimental verification 3: diffusion between bromine gas & air
- Experimental verification 1: diffusion in liquids
- Experimental verification 1: diffusion in solids
- Conclusions
For detailed conceptual understanding of diffusion, click on the link: https:/diffusion-and-kinetic-particle-theory/
Experimental Verification 1: Diffusion between Hydrogen Gas & Air
Following experiment provides evidence related to diffusion between hydrogen gas and the air.
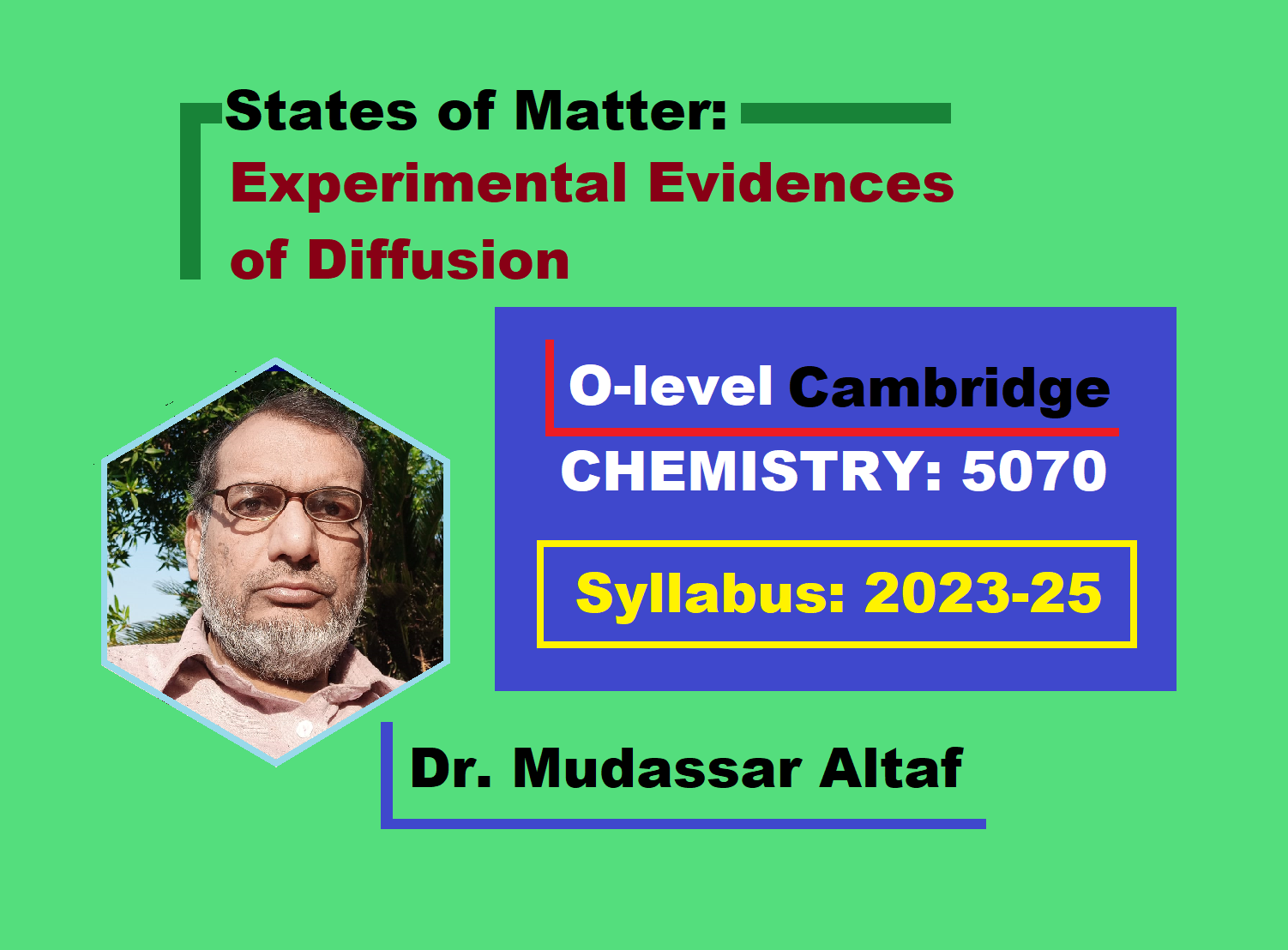
Apparatus Setting:
- Make a hole in the middle of a card and pass it through manometer tube. Hold this card with the help of rubber stopper through which the tube is passing. Place the card on an iron stand support.
- Now, place a porous cylinder on the card. Also close the top of cylinder with another piece of card. This porous cylinder has tiny channels in its walls across which the molecules can pass.
Experiment:
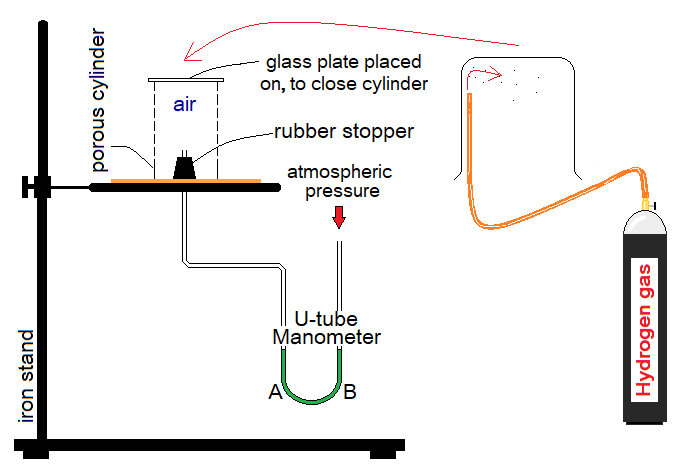
- Fill the U-tube of manometer with green water. Both of its arms, A & B will have the same level of green water initially.
- Now, fill a beaker with hydrogen gas from the cylinder or can be prepared in the lab. The hydrogen gas is lighter than air; so, the beaker is kept inverted. Immediately take the filled inverted beaker towards porous cylinder and cover that all inside the beaker.
Observations:
- Inside the porous cylinder, there is air. Hydrogen is lighter than air; so, it will diffuse-in faster than air. Relatively, the air will diffuse-out slow. This will develop a pressure of mixture of gases inside the manometer tube; so, the green liquid in arm A will fall down, while, will rise up in arm B.
- Soon after, the equilibrium state will be established by the homogeneous mixture of air and hydrogen gas to make the levels same of green water in A & B.
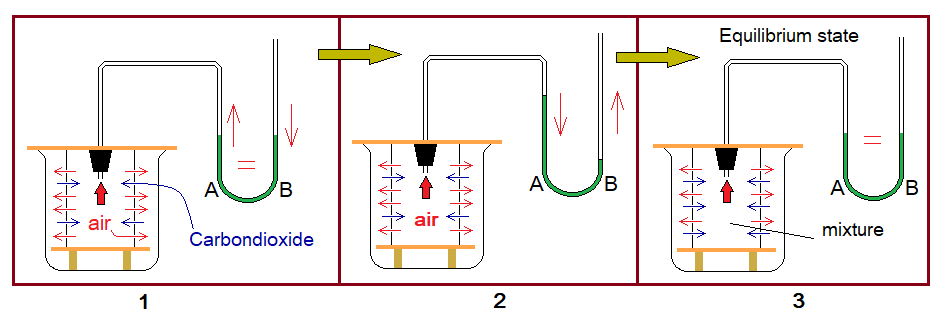
Experimental Verification 2: Diffusion between Carbondioxide & Air
Following experiment provides evidence related to diffusion between carbondioxide gas and air.
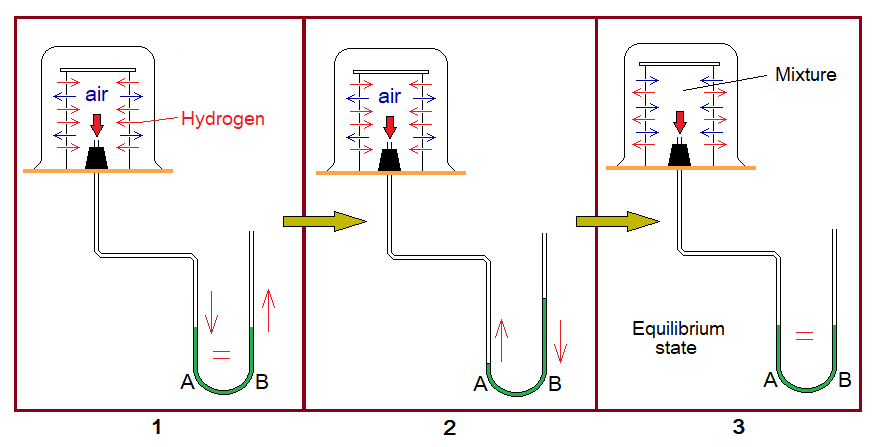
Apparatus Setting:
Almost same as in experiment 2 with slight differences. Get the concept by the following diagram.
Experiment:
Now, fill a beaker with carbondioxide gas from the cylinder or can be prepared in the lab. This gas is heavier than air; so, the beaker is not kept inverted. Immediately move the set apparatus of porous cylinder and manometer towards the filled beaker.
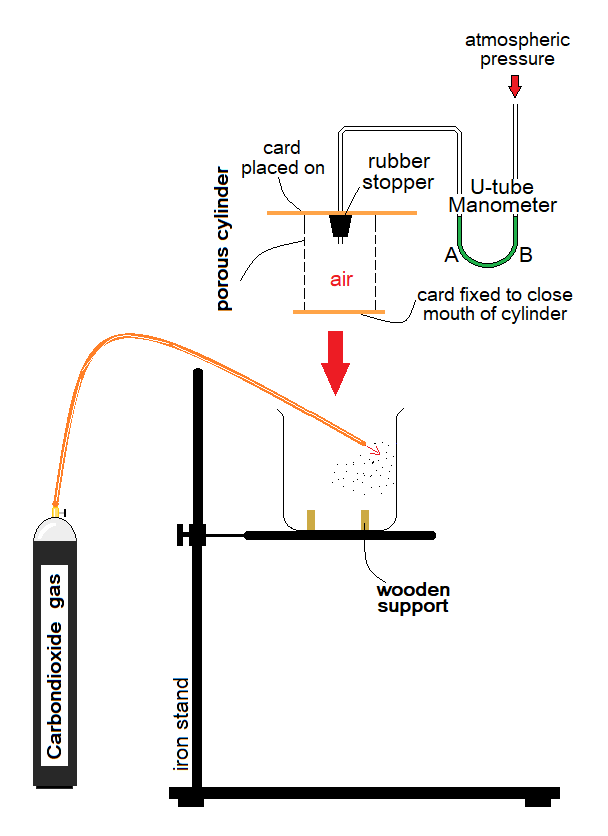
Observations:
- Inside the porous cylinder, there is air. Carbondioxide gas is heavier than air; so, it will diffuse-in slower than air. Relatively, the air will diffuse-out more rapidly. This will develop a reduced pressure of mixture of gases inside the manometer tube; so, the green liquid in arm A will rise up; while, will fall down in arm B.
- Soon after, the equilibrium state will be established by the homogeneous mixture of air and carbondioxide gas to make the levels same of green water in A & B.
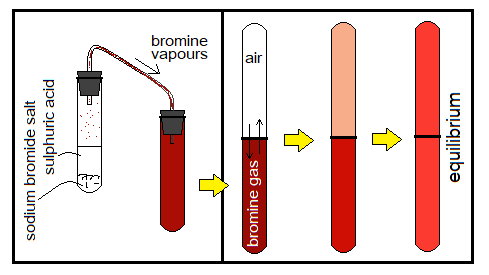
Experimental Verification 3: Diffusion between Bromine Gas & Air
Following is another lab experiment that provides evidence related to diffusion between bromine gas and air.
Take bromine gas in a test tube and invert another empty tube on it containing air. The colour of bromine gas is dark reddish-brown. After some time, this colour will fade in lower test tube due to mixing of air into bromine and vice versa. The colourless appearance of upper test tube will begin to change into shade of bromine gas gradually. Thereafter, a uniform shade of the colour will be appeared in both test tubes due to natural mixing by diffusion.
How bromine gas is made in lab? If concentrated sulphuric acid is poured into a test tube containing some salt of sodium bromide (white crystalline powder), then as a result, reddish-brown vapours of bromine gas are produced.
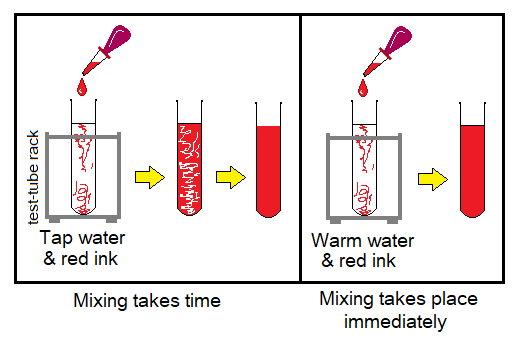
Experimental Verification 1: Diffusion in Liquids
Following is a simple experiment that provides evidence related to diffusion in liquids.
- Take tap water in a test tube and drop few drops of ink in it. Any colour of the ink can be used. Allow the test tube mixture to stand for certain period of time. The ink colour will make the uniform-coloured solution due to mixing by diffusion. Diffusion takes time in cold water as compared to hot water.
- Now, take warm water in another test tube and drop few drops of ink in it. Note that it will be mixed to form a uniform-coloured solution immediately. This experiment also shows that the diffusion rate increases on increasing the temperature.
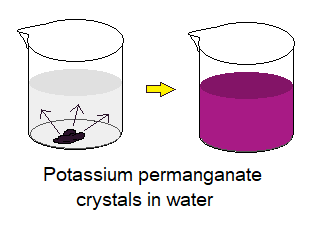
Experimental Verification 1: Diffusion in Solids
Following is a simple experiment that provides evidence related to diffusion in solids.
Take tap water in a beaker and add few crystals of potassium permanganate. These crystals are extremely dark purple, even look blackish in shade. The crystals will start dispersing into liquid and after certain period of time a purple-coloured solution will be appeared. Behind the theme, there is a concept of solid diffusion in liquid. Although, it is slow in its rate as compared to liquid-liquid diffusion, but, doing with the same phenomenon. The dispersion of crystals’ particles is from higher to lower of their concentration, until making the dispersion uniform.
Conclusions:
The experiments provide the following evidences:
- Diffusion takes place in all the three states of matter.
- The rate of diffusion is slow in solids, medium in liquids, while fast in gases.
- Diffusion takes place from higher concentration of particular particles to the area where their concentration is lower. This phenomenon can be explained in all of the above experiments.
- The end result of dispersion by diffusion is making a uniform mixture throughout; and that is an equilibrium state.
- Dispersion also provides evidence that the matter is composed of tiny particles.