Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Shells or orbits
- Radius of shells from the nucleus
- Number of electrons in shells
- Types of shells
- Electronic configuration
Shells or Orbits:
The shell is recognized as energy level around the nucleus of an atom where electrons revolve. There are different energy levels, seven, around the nucleus, recognized as K, L, M, N, O, P & yet finally the Q.
The orbits are the imaginary circular paths where electrons revolve. A shell is the energy level of an orbit. An orbit is also recognized as K, L, M, N, O, P, and yet finally the Q with respect to their energy levels. So, the terms shell or orbit are interchangeable.
The shells have their own energy levels. Closer a shell to the nucleus, lesser will be its energy level and vice versa. The shells have their own particular n-number as: K=1, L=2, M=3, N=4, O=5, P=6 & Q=7. The K has lowest energy level and its n-number is ‘1’; L is second one after K and having next higher energy level and its n-number is ‘2’; and so on towards Q having ‘n=7’ the highest energy level.
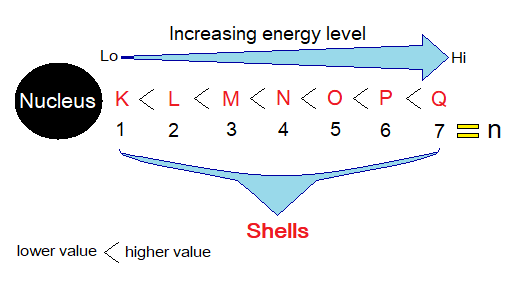
Radius of Shells from the Nucleus:
The very first shell around the nucleus is K, then comes L and the last one is 7th the Q far away from the nucleus, as shown in the figure. Thus, K has lowest radius while Q has highest relatively.
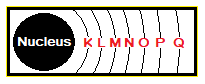
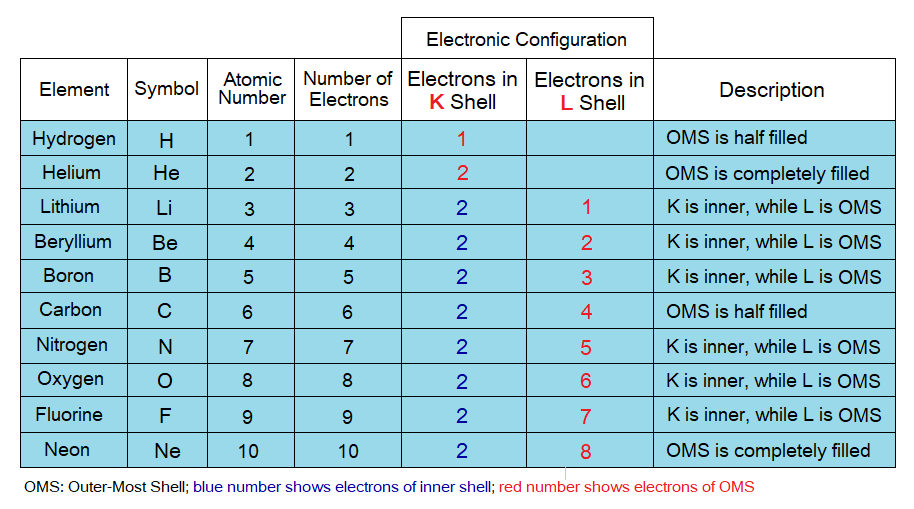
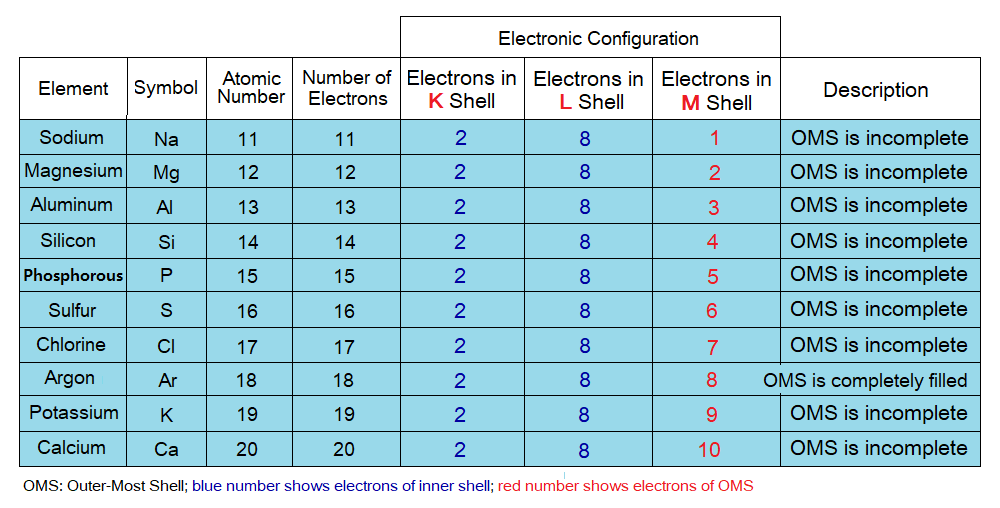
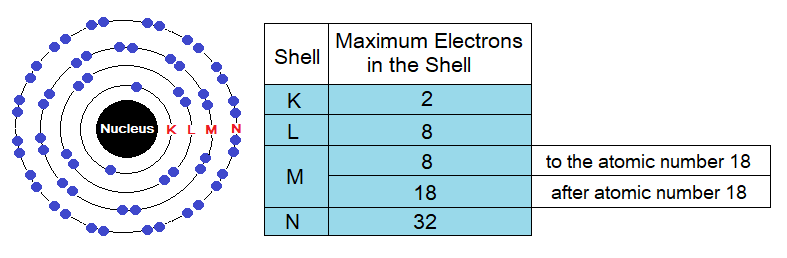
Number of Electrons in Shells:
Maximum number of electrons that can be occupied in different shells are different, detail is as under in the table and the diagram. The electrons might be lesser to the maximum capacity of a shell but cannot exceed the number to that capacity. For example, K shell has maximum capacity of two electrons to be accommodated, but the third electron can never be occupied here; likewise, for all other shells. So, by that way the third electron will go to next energy level to accommodate, i.e., to L shell.
Exercise 1:
Think where the 29th electron will go to accommodate in the shell, name the shell.
Types of Shells:
There are two types of shells in an atom:
- Inner shells: The shells in-between last (OMS) shell and the nucleus are called inner shells. These are completely filled by electrons.
- Outermost shell (OMS): This is the last shell of an atom and might be completely filled, half-filled or more or less than half-filled by electrons, depending upon the remaining electrons of a particular atom after filling inner shells. The chemical reactions, chemical bonding, cation or anion formation take place in OMS.
Hydrogen and helium are the only atoms having no inner shell; because, their OMS is K-shell due to lesser number of electrons they have and can be accommodated in K according to the shell’s maximum capacity.
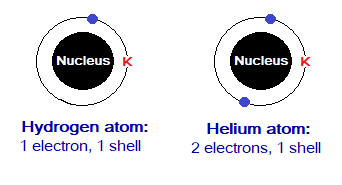
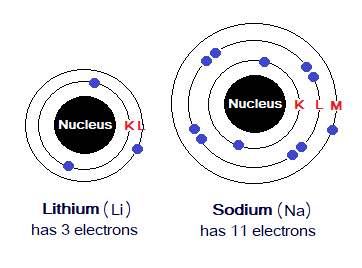
Exercise 2:
- Looking into the following diagrams, come to know how electrons are distributed in various shells of Li & Na?
- Why both the atoms have only 1 electron in their outermost shell?
- Justify, Li has only 1 inner shell, while Na has 2.
Electronic Configuration:
Configuration means ‘arrangement, pattern’. Thus, electronic configuration can be defined as, “the distribution of electrons in the orbits of an atom”. Following points should be considered while making electronic configuration.
- Must come to know the number of electrons of an atom of an element. Remember, the atomic number is the number of electrons in a particular atom. Because, in neutral atom the number of electrons is always equal to the number of protons.
- Keep in mind the maximum capacity of the shells to accommodate the electrons.
- First inner shells will be filled and fill-in the electrons in the sequence of K, L, M, N, O, P and lastly, the Q.
- Remember, the inner shells will be filled completely to their maximum capacity. However, the outermost shell (OMS) not necessarily be filled completely depending upon the available remaining electrons.
Let’s study the oxygen atom as an example. The atomic number of oxygen atom is 8, thus, there are 8 electrons in its shells. The atomic number is written by ZE, can go through from the link https:/neutrons-atomic-mass-mendeleev-periodic-table-foundational-principles/ . The K-shell will be filled first, an inner shell, by two electrons. The remaining 6 electrons will accommodate in L-shell, the outermost shell (OMS) of oxygen. Pervade through the following tables to learn. The electronic configuration by means of shells is written by the following ways:

Exercise 3:
Write electronic configuration of elements on the patterns of oxygen by using the following tables.
Knowledge in Advance:
The OMS of oxygen will not be filled completely to its maximum capacity of 8 electrons due to unavailability of more electrons to this atom. This is the reason why ions are formed; because, oxygen will get two more electrons from another atom to complete its OMS; but, by doing this, it will gain an ionic configuration, i.e., O-2.