Dr. Mudassar Altaf, Associate Professor, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
- Definition
- Overall reaction
- Mechanism of chemical change
- A brief account of chemicals
Definition:
The aliphatic aldehydes or ketones show condensation reaction with aromatic aldehydes to yield alpha-beta unsaturated aldehyde or ketone.
Overall Reaction:
The mechanism of chemical change takes place in the presence of alkali or an acid. For example, a reaction between an acetone and benzaldehyde. This is solvent free mechanism in the presence of solid NaOH in mortar-pestle and completes in 2-minutes. Resultantly, benzylidene acetone and bis-benzylidene acetone are produced by two moles of acetone and three moles of benzaldehyde in the overall reaction.
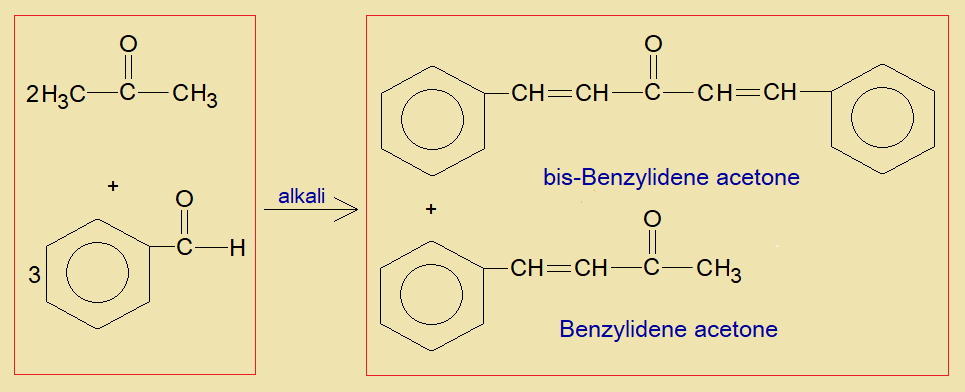
Mechanism:
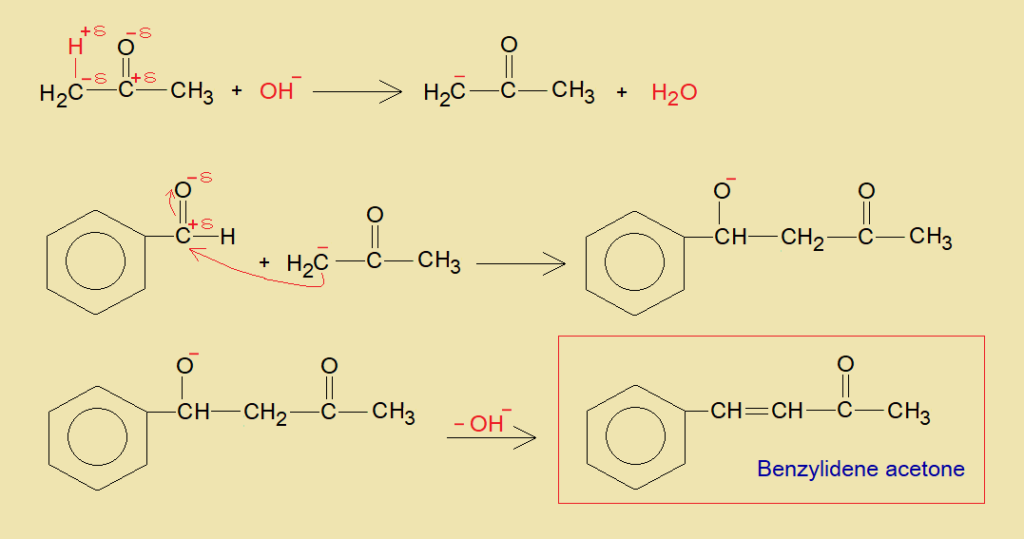
- Beta-carbon of acetone makes carbanion by eliminating hydron (H+) that makes water with hydroxide ion of an alkali.
- The carbanion acts as active methylene, attacks on partial positive carbon of benzaldehyde; and generates a C-C linkage.
- The carbonyl oxygen of benzaldehyde attains negative charge; it removes as hydroxide ion with hydrogen of beta-carbon of acetone and establishes a double bond between two carbons. Ultimately, benzylidene acetone is formed.
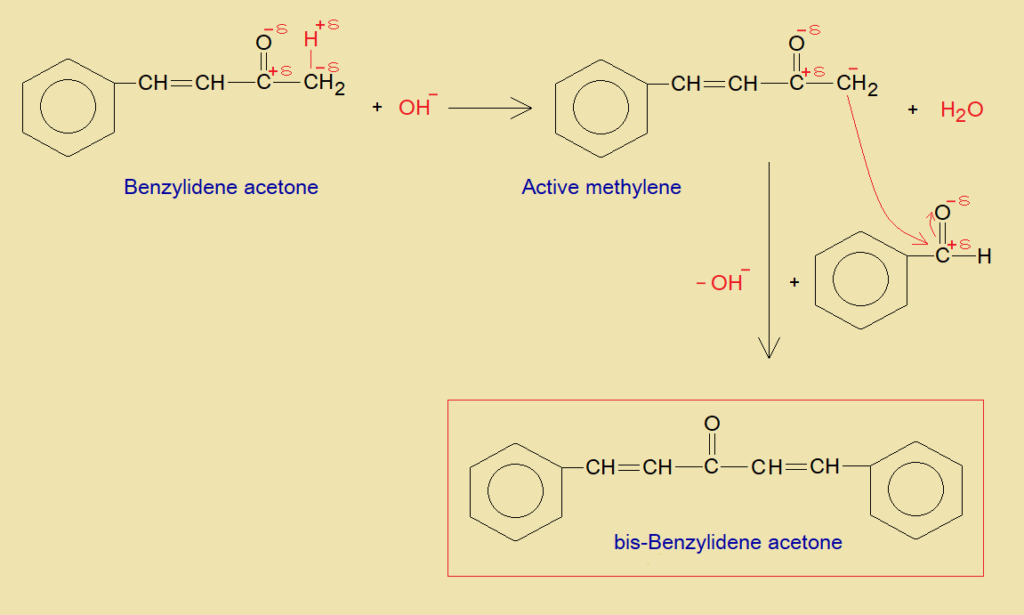
- The terminal beta-carbon of benzylidene acetone undergoes into similar chemical change and produces bis-benzylidene acetone with another benzaldehyde, as explained in the following mechanism. Bis is a Latin word meaning ‘twice’. On either side of acetone two carbenes of benzylidene groups are attached. Benzylidene is C6H5CH; where carbon of CH is carbene having two unshared valence electrons. This carbene makes unsaturation with beta-carbon of acetone.
A Brief Account of Chemicals:
Acetone: It is a colorless flammable organic laboratory liquid reagent, having fruity but pungent smell. Its m.pt. is -94.9°C and b.pt. 56°C. It is miscible with water by all proportions, also miscible with organic solvents. The vapors of acetone are dissolved in air humidity and can be explosive. Acetone is also used as organic solvent in labs.
Benzaldehyde: It is a colorless liquid, having almond odor. Its melting point is -57°C and b.pt. 178°C. Its solubility in water in less than 7 g/dm3 at room temperature. It is a flavoring agent in food due to almond odor and recommended safe in food; also used in cosmetics.
NaOH: It is white odorless ionic crystalline solid. Also called caustic soda or soda lye (lye means a strong alkaline solution). At room temperature, NaOH is highly soluble in water, i.e., 1000 g/dm3 and increases on increasing temperature. The water solution having no odor and is colourless in its appearance. The solubility in water is exothermic and is dangerous in terms of splashing. It forms several hydrates, mono, di, tri, tetra, penta, hepta and even trihemihydrate (3.5 water of crystallization). Its pellets are hygroscopic in nature and readily dissolve in moisture.



