Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Chemical energetics: definition
- Types of energy changes: exothermic and endothermic
- Enthalpy etymology
- Energy of activation: definition
- Energy of activation in exothermic reactions
- Energy of activation in endothermic reactions
- Examples of exothermic reactions
- Examples of endothermic reactions
Chemical Energetics:
Definition: It can be defined as, “the branch of chemistry that deals with the energy changes during physical or chemical changes”.
Types of Energy Changes:
There are two types of energy changes, exothermic and endothermic.
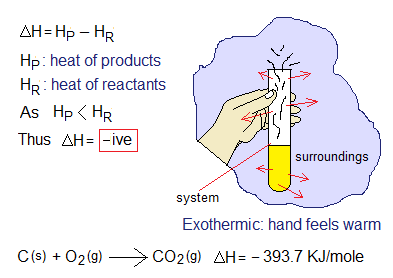
1. Exothermic Reactions: The chemical reactions in which the energy is released are called exothermic. Exo means ‘outward’; and thermic has its Greek origin from the word ‘thermos’ meanings ‘heat’. Enthalpy is another synonym of heat having its Greek origin from the word ‘enthalpein’ meaning ‘heating’.
The heat change (enthalpy change) is denoted by ∆H. It is negative for exothermic reactions. Because, the heat contents of reactants are greater than products. So, the excess heat is evolved. The system releases heat into the surroundings; hence, the test tube will feel warm to the hand. The burning of carbon (coal) in the presence of oxygen gas produces heat; this combustion reaction releases 393.7 kilojoules per mole heat.
Candle wax is an organic compound; and when it burns in the presence of atmospheric oxygen, it releases heat, causing the surroundings warm by rising temperature.
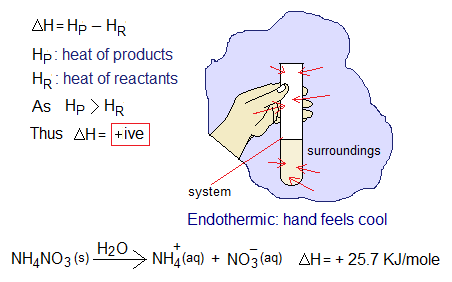
2. Endothermic Reactions: The chemical reactions in which the energy is absorbed are called exothermic. Endo means ‘inward.
The heat change (∆H) is positive for endothermic reactions. Because, the heat contents of reactants are lesser than products. So, the system absorbs heat from the surroundings; hence, the test tube feels cold to the hand. When ammonium nitrate is dissolved in water, it absorbs heat from surroundings; per mole of ammonium nitrate absorbs 25.7 kilojoules heat.
Making brine (salt water by NaCl) is an endothermic reaction, it causes the temperature of immediate surroundings down.
Energy of Activation:
Definition: “The minimum amount of energy that is required to change reactants into products”. It is denoted by Ea.
The Ea follows the successful collision between particles of the reactants that leads to break the old bonds and make new ones, called activated complex; and it is the transition state. The origin of the ‘transition’ is Latin from the word ‘transitio’ meanings ‘go across, going from one side to another; likewise, in chemistry, going away from one state and moving to another; in other words, physical or chemical change’.
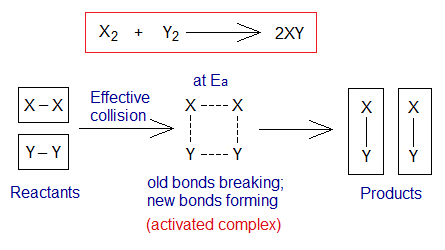
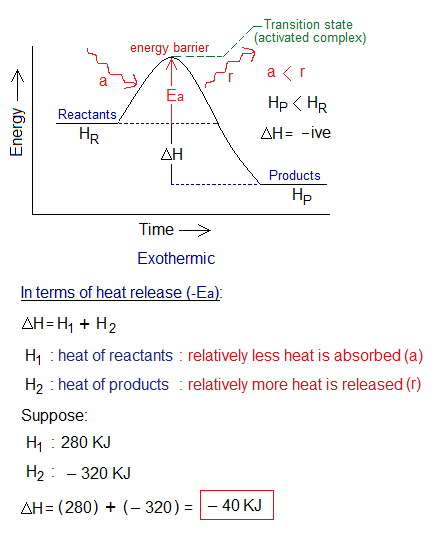
1. Ea for Exothermic Reactions: The minimum amount of energy is the energy of activation, making activated complex. It is also called the energy barrier required for reactants to change into products. At highest peak of energy barrier, the Ea is the minimum energy required for chemical change.
Although, exothermic reactions release energy at the end. But first they need some external source to start like to heat, to ignite or by means of a catalyst or can also produce Ea by effective collisions. The existing bonds are broken. So, the energy of the system is increased in terms of ‘activation energy’; it is called transition state (high energy state). However, when new bonds are formed, then the system releases energy; or in other words, Ea used up and eventually, comes to settle at the heat contents (enthalpy) of the products. Relatively, more heat is released as compared to absorbed in exothermic reactions.
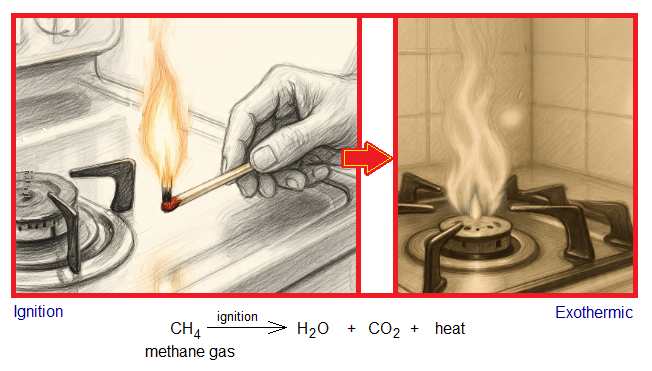
Burning of a gas in the stove is an example. There is a need of an external source to light up the flame in the stove by using match stick or an electric spark. Then, stove gas starts burning the in the presence of atmospheric oxygen and releases heat; so eventually becomes exothermic chemical reaction.
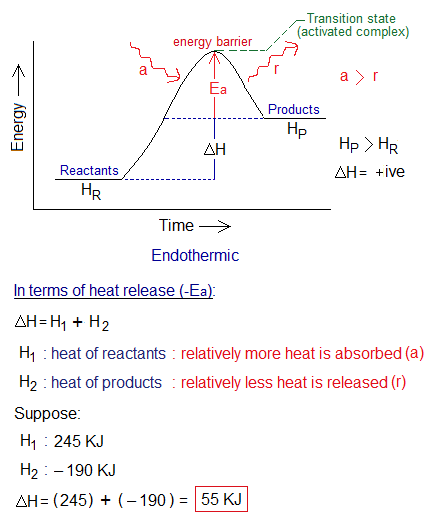
2. Ea for Endothermic Reactions: The minimum amount of energy is the energy of activation, making activated complex. As mentioned, that Ea is the energy barrier required for reactants to change into products. Again, in endothermic reactions, at highest peak of energy barrier, the Ea is the minimum energy required for chemical change.
Endothermic reactions need energy for their completion. Additionally, when pre-existing bonds are broken, energy of the system increases in terms of ‘activation energy’; it is called transition state (high energy state). However, when new bonds are formed, then the system releases energy; or in other words, Ea used up and eventually, comes to settle at the heat contents of the products. Relatively, less heat is released as compared to absorbed in endothermic reactions.
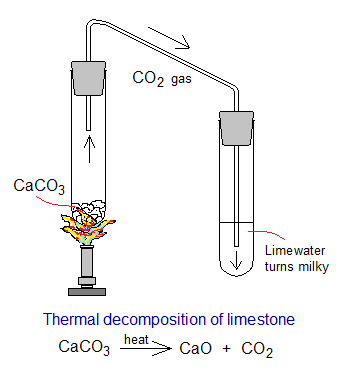
Thermal decomposition of limestone (CaCO3 calcium carbonate) produces quicklime (CaO calcium oxide or burnt lime) and carbon dioxide gas. In a lab when CaCO3 is burnt in a test-tube and the gas produced is allowed to pass through limewater [Ca(OH)2], it turns milky; making confirm the formation of CO2 gas. It is endothermic reaction.
Examples of Exothermic Reactions:
1. Dissolution of CaCl2 in water: It is exothermic when calcium chloride is dissolved in water at room temperature.
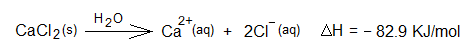
2. Burning of methane gas (natural gas) in the presence of oxygen of air.
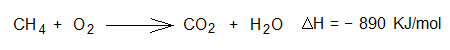
3. Neutralization reaction between acid and base: A reaction between aqueous solutions of hydrochloric acid (HCl, suppose 1 mole) and base sodium hydroxide (NaOH, suppose 1 mole) at room temperature.
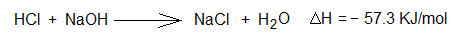
4. Common examples: Many examples exist in our daily life observations that are exothermic in nature. Burning of a paper, wood, candle; during respiration: the burning of glucose in our cells in the presence of oxygen that we breath-in, burning of petrol/diesel in automobile engines, rusting of iron etc.
Examples of Endothermic Reactions:
1. Dissolution of NaCl & NH4Cl in water: It is endothermic when table salt is dissolved in water at room temperature. Likewise, the dissolution (to dissolve) of ammonium chloride salt in water is also endothermic.
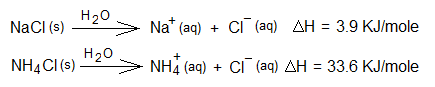
2. Photosynthesis: Plants make glucose and oxygen by using carbon-dioxide and water in chlorophyll in the presence of sunlight energy. This phenomenon is called photosynthesis and is opposite of respiration.
3. Boiling a liquid: There is a need to change the phase of liquid into gas by means of heating the liquid. It is necessary to overcome the intermolecular attractive forces among molecules in liquid phase by external source of heat.
4. Solid to liquid melting: The phase change from solid to liquid absorbs heat from the surroundings; for example, when ice is kept in a tray under sunlight, it will melt indeed. The melting of glaciers in summer season.