Dr. Mudassar Altaf, Associate Professor, Higher Education Department, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Three states of matter and plasma
- Kinetic Particle theory, phase change, and kinetic energy
- Solid to liquid phase change, melting point, freezing point
- Liquid to gas phase change, boiling point, condensation point
- Importance of melting and boiling points
Three States of Matter:
There are three states of matter, solid, liquid and gas; although, the plasma is also considered its fourth state. Here, in this article, the interconversion of three primary states of the matter will be discussed.
Kinetic Particle Theory (Kinetic Theory of Matter):
This theory describes how the kinetic energy of the matter (particles) is involved to change the state of the matter.
The change of the state of matter is called phase change and it is a physical phenomenon. The term ‘phase’ has its origin from Greek word ‘phasis’ meaning ‘appearance’. So, phase means the appearance of the matter, like solid, liquid or gas.
Kinetic energy is the energy due to motion of the particles. This energy is involved to increase or decrease the movements of the particles by raising or lowering the temperature respectively. By doing this, the intermolecular forces between particles decrease or increase to bring about the phase change.
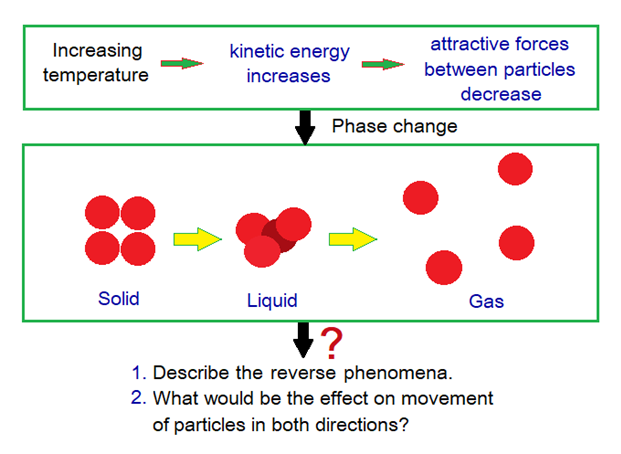
Solid to Liquid Phase Change:
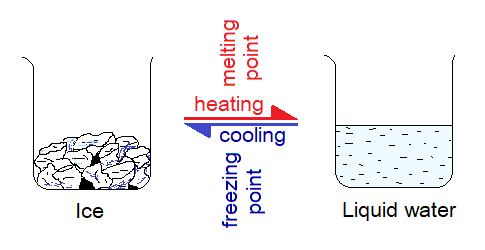
When solids are heated, they absorb heat and melt. For example, the ice melts when absorbs heat; the wax of candle melts when absorbs heat. So, there is a need of energy (heat) that is provided to the particles that increases their kinetic energies and reduces their forces of attraction; resultantly, their mobility increases. The temperature at which a solid substance melts is known as its melting point. It is defined as, “the constant temperature at which a pure solid substance changes its phase (appearance) to a liquid at standard atmospheric pressure”.
The standard atmospheric pressure is 1 atm that is measured at sea level. The melting and boiling points are conditional on lowering or raising of atmospheric pressure. Due to conceptual limitations, the topic has not been discussed here.
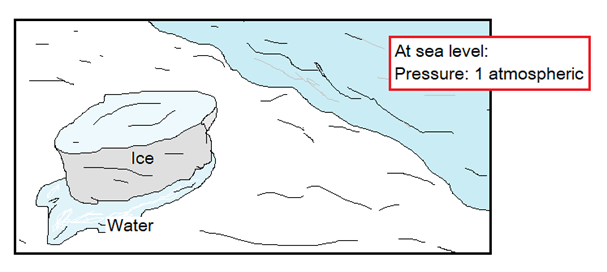
The melting point of pure ice is 0°C at 1 atmospheric pressure. C for Celsius or Centigrade scale. On other scales, at Fahrenheit (F) and Kelvin (K) the equivalence would be as:
0°C = 32°F = 273.15K
It should be heeded upon that the degree sign (o) is not used with Kelvin scale (aka absolute scale). Melting point and freezing points are same terms and having same values. However, the freezing point can be defined as, “the constant temperature at which a pure liquid substance changes its phase to a solid form at standard atmospheric pressure”.
Melting point = Freezing point
Liquid to Gas Phase Change:
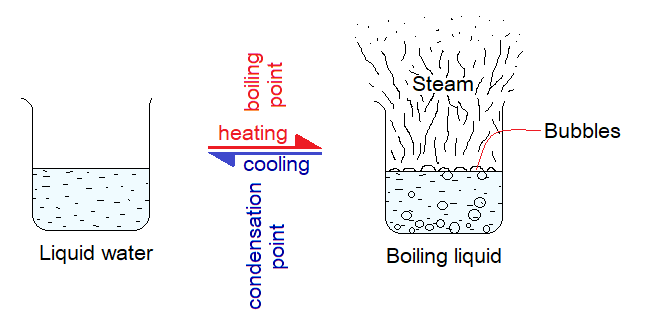
When liquids are heated, they absorb heat and get changed into gaseous phase. For example, the water forms steam when absorbs heat. So, there is a need of energy that is provided to the particles that increases their kinetic energies and reduces their forces of attraction; so for that reason, their mobility increases. The temperature at which a liquid substance forms gas is known as its boiling point. In simple terms, it is defined as, “the constant temperature at which a pure liquid substance changes its phase to a gas at standard atmospheric pressure”. It is a property of a liquid to form bubbles when boils. The boiling point of pure water is 100°C at 1 atmospheric pressure. On other scales, it would be:
100°C = 212°F = 373.15K
Boiling point and condensation points are same terms and having same values. However, the condensation point can be defined as, “the constant temperature at which a gaseous phase of a pure substance changes to its liquid form at standard atmospheric pressure”.
Boiling point = Condensation point
Importance of Melting & Boiling Points:
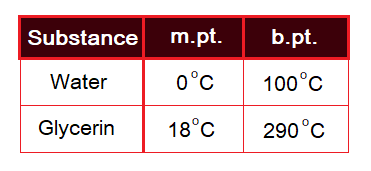
Different substances have different values of their melting (m.pt.) and boiling points (b.pt.), even at same condition of atmospheric pressure. The m.pt. and b.pt. are used as test in chemistry laboratories for the determination of unknown compounds using reference table. For example, at 1 atm the values for water and glycerin are as under: