Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Atomic number and periodic table
- Atoms are neutral particles as a whole
- What happens when subatomic particles are changed?
Atomic Number and Periodic Table
By definition, “the number of protons of an atom of an element is called atomic number”. The atomic number is written by a symbol ‘Z’; and aka Proton Number.
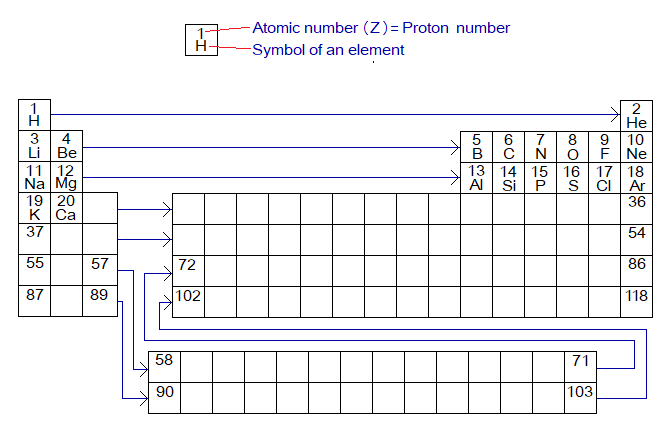
Different elements are different from each other due to having differences in the number (quantity) of their subatomic particles: protons, electrons and neutrons. The main identity of an atom is its atomic number; in other words, counting the number of protons that exist in a particular type of an atom. Henry Gwyn Jeffreys Moseley (1887-1915) formulated Moseley Law of Modern Periodic table by which he arranged elements in the table by increase in their atomic numbers one-by-one from left to right. Henry Moseley was a British physicist selected in British army as Signals engineer and died in World war I just at the age of 27. Below to each element, he placed another element similar in its properties to the above one. For 118 elements, look through the following periodic table and count atomic number 1 to 118 by going left to right. The detailed features of periodic table don’t have a need of discussion here; the main concern is atomic numbers and their placement in a particular order for the elements’ disposition according to the similarities in their properties.
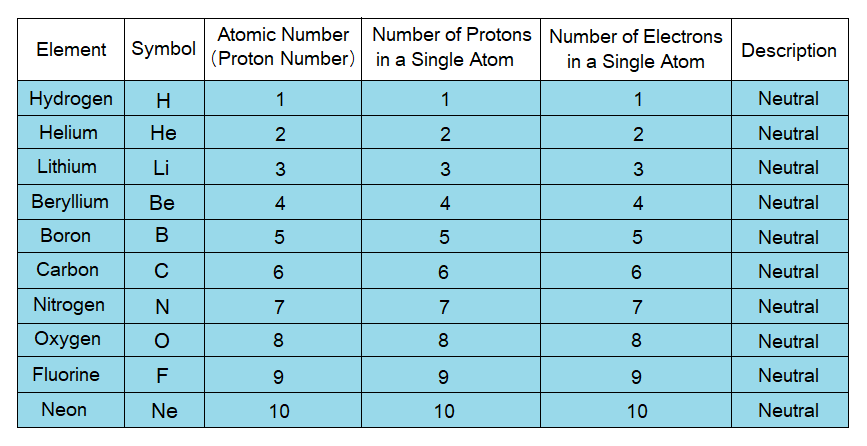
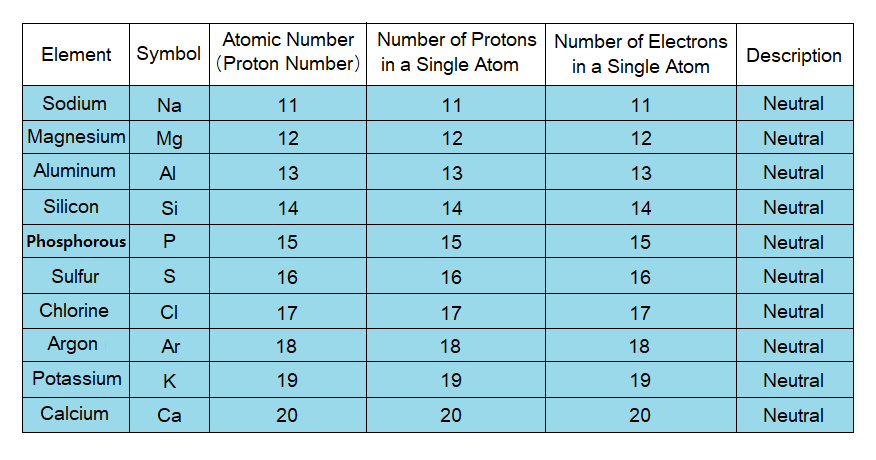
Atoms are Neutral Particles as a Whole:
Amongst subatomic particles, protons are positively charged, while electrons are negatively charged. The number of positively as well as negatively charged particles is same in atoms, thus, making them neutral as a whole. For example, in Lithium there are 3 protons and 3 electrons; so, it is neutral. In another example of Boron there are 5 protons and 5 electrons, making the atom neutral as a whole. Similar is the case with all other atoms.
What Happens When Subatomic Particles are Changed?
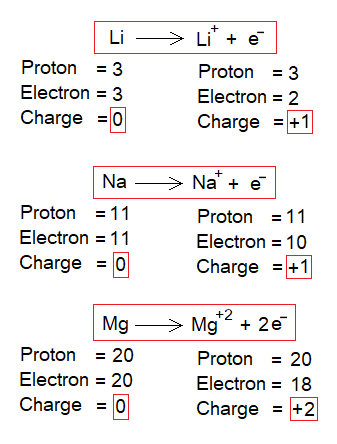
- When an electron is removed from a neutral atom then the atom attains a positive charge called a cation. However, the atom remains the same. For example, lithium remains lithium but changes to cation. Likewise, same is the case with all other atoms of any kind of element.
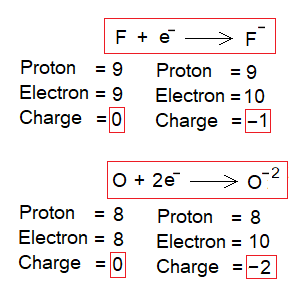
- When an electron enters into a neutral atom’s shell then the atom attains a negative charge called an anion. However, the atom remains the same. For example, fluorine remains the same but changes to anion. Likewise, same is the case with all other atoms of any kind of element.
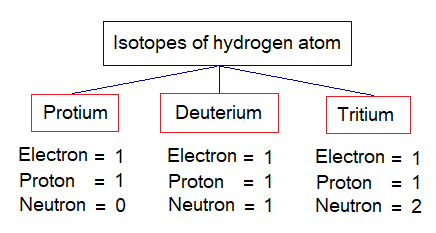
- The similar atoms if are different from one another due to dissimilarity in the number of their neutrons then these atoms are called isotopes of the same element. For example, isotopes of hydrogen and look through the change in the number of their neutrons.
- However, the point of concern is that the main identity of the atom is its proton number (atomic number). When there is a change in proton number then the atom doesn’t remain the same; in other words, the element itself changes into another element. This is a nuclear delay process and can’t be achieved by chemical means.