Dr. Mudassar Altaf, Associate Professor, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Definition
- Overall chemical reaction
- Brief mechanism
- Comprehensive mechanism
- A brief account of the chemicals
Definition:
The addition of an aryl group (-Ar) to a methylene group (-CH2-) of an active methylene compound is called arylation.
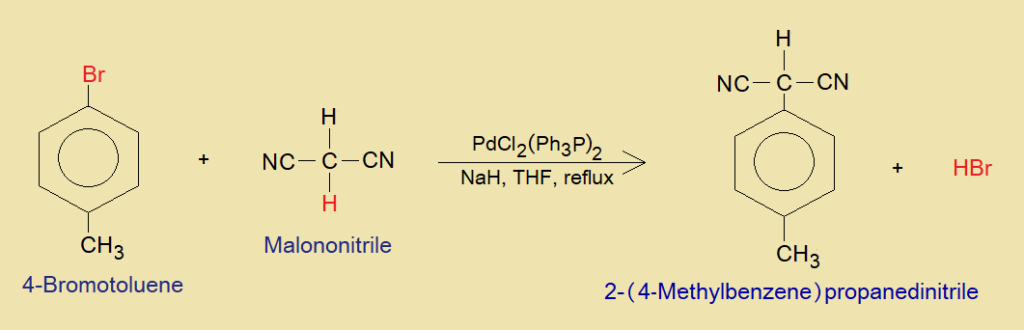
Overall Chemical Reaction:
The product is formed by the arylation of malononitrile with 4-bromotoluene by reflux. Tetrahydrofuran is used as solvent; and Palladium complex is used as cross-coupling reagent during the chemical change.
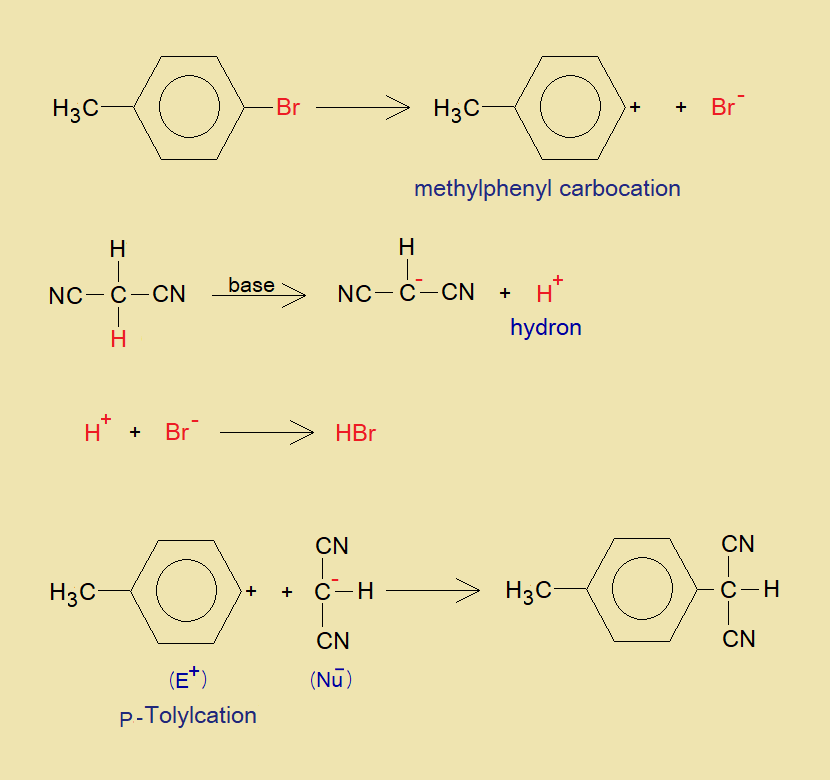
Brief Mechanism:
The mechanism without considering the involvement of cross-coupling reagent [PdCl2(Ph3P)2] is so simple, as the 4-bromotoluene makes methylphenyl carbocation (p-Tolylcation) by debromination of bromide ion, and malononitrile makes carbanion by dehydronation (H+). Both the nucleophile and electrophile undergo into arylation and ultimately the product, 2-(4-methylbenzene) propanedinitrile, is formed. The detailed mechanism is concerned with the involvement of cross-coupling complex [PdCl2(Ph3P)2].
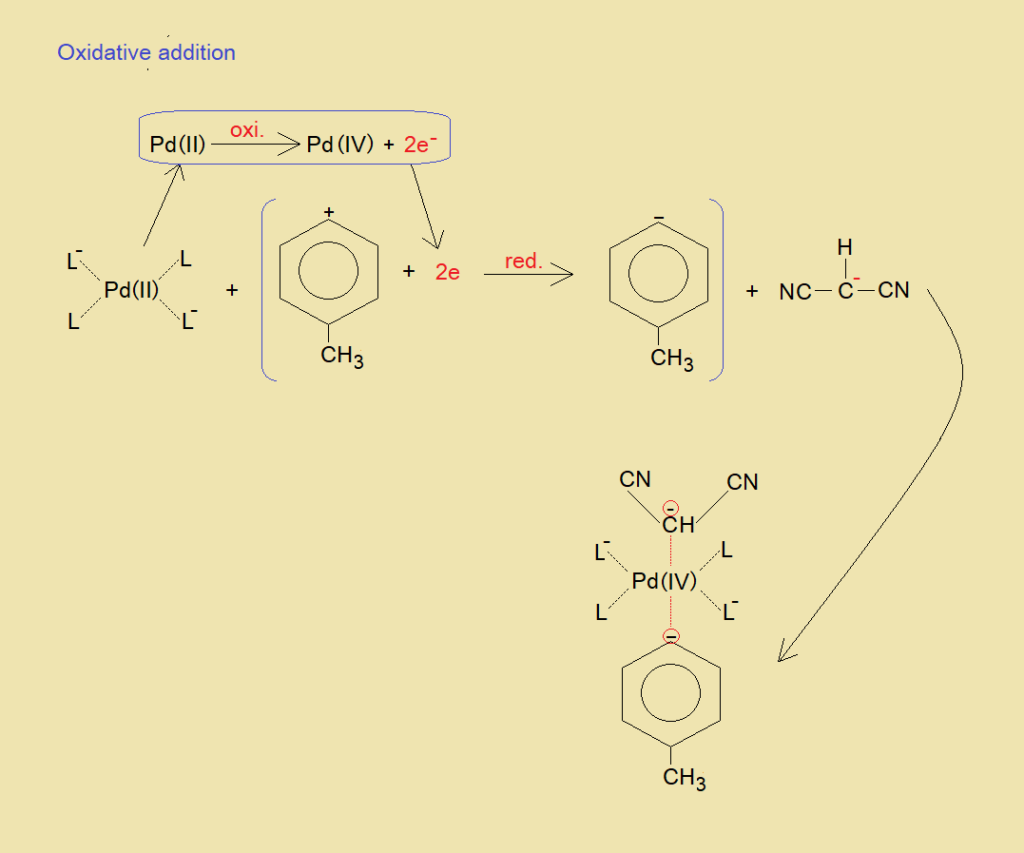
Comprehensive Mechanism:
The detailed mechanism is a proposed scheme that describes both the steps involved, oxidative addition and reductive elimination.
a. Oxidative Addition:
In this step the Pd catalyst undergoes into increase in its oxidation state from +2 to +4 by means of oxidation. Further, its coordination number also increases from 4 to 6. Thus, makes a complex with two newly formed ligands, one nucleophile of malononitrile; while the other electrophile p-tolylcation reduces by two electrons oxidized by Pd and forms another nucleophile p-tolylanion. Ultimately, a cross-coupling complex is generated, as shown in the following equation.
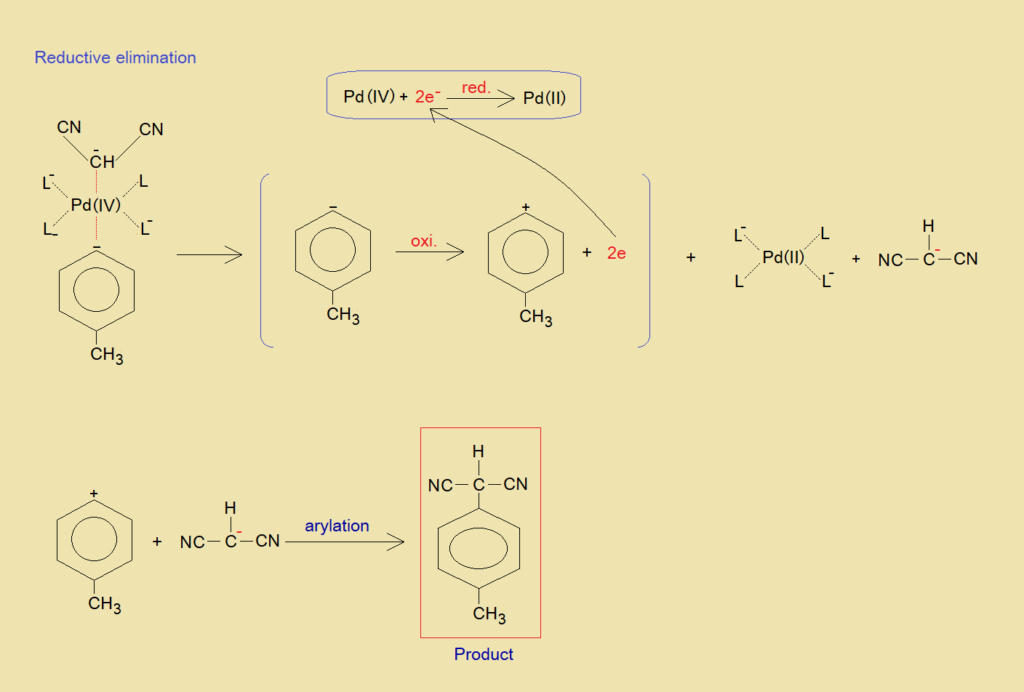
b. Reductive Elimination:
This phase is reverse of all steps played during oxidative addition. Again, Pd attains its previous oxidation state +2 by reduction. The p-tolylcation is formed again by oxidation. Thus, at the end, the nucleophile of malononitrile is arylated by p-tolylcation to generate the final product of 2-(4-methylbenzene) propanedinitrile.
A Brief Account of the Chemicals:
Malononitrile is a white crystalline powder, having melting point 32°C. It is commonly used in pharmaceutical industry. Propanedinitrile; dicyanomethane; malonodinitrile etc. are its various names.
4-bromotoluene is a white crystalline solid but having m.pt. just 29°C; it appears as pale-yellow clear liquid having boiling point at 184°C. It has other names like p-Tolylbromide; 4-Tolylbromide; 4-Bromo-1-methylbenzene; 1-Methyl-4-bromobenzene; 1-Bromo-4-methylbenzene; p-bromotoluene etc. It is used as organic solvent and thinner in paints etc.
Tetrahydrofuran is written as THF. It is water soluble organic colourless liquid; having boiling point 66°C. THF is a cyclic ether and is used as organic solvent. The other names are Oxolane; 1,4-Butyleneoxide; 1,4-Epoxybutane; Tetramethylenoxide etc.
NaH is a white or grey powdered inorganic compound that is used in many organic syntheses. It is insoluble in any solvent. It is highly dangerous in the sense that it ignites in humidity vigorously; thus, the dispersion of its powder form is available in mineral oil for safety purposes.
PdCl2(Ph3P)2 is an organopalladium complex, available in yellow powder form; having melting point 260°C. It has various names, like the common one is, Bis(triphenylphosphine)palladium(II)dichloride. The complex is used in many Palladium catalyzed organic coupling reactions involving C-C coupling such as Suzuki reaction, Kenkichi Sonogashira coupling reaction. During the mechanism of catalytic cycling, the oxidative addition and reductive elimination take place. The structural formula is shown below.




