Mechanism of Chemical Change
Dr. Mudassar Altaf, Associate Professor, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Definition
- Overall reaction
- Mechanism of chemical change
- A brief account of chemicals
Definition:
The addition of an alkyl group (-R) to a methylene group (-CH2-) of an active methylene compound is called alkylation.
Overall Reaction: A general mechanism of the chemical reaction is shown here.
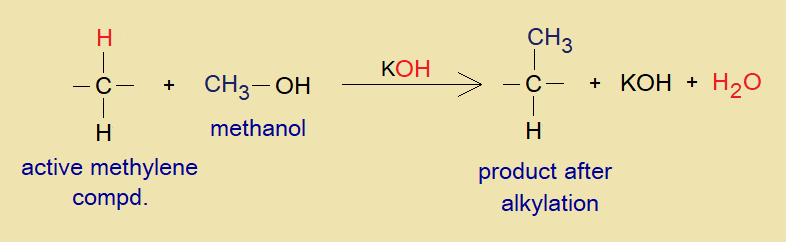
By means of deprotonation of active methylene compound with the help of a base the alkyl radical (R+) is substituted onto the position of hydron (H+), resultantly, the alkylation occurs.
Mechanism of Chemical Change:
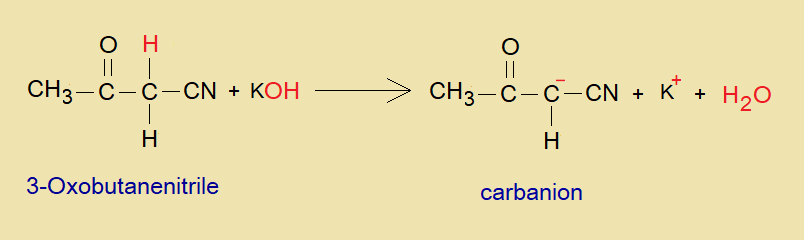
The comprehensive chemical change has been elucidated here for an alkylation of 3-oxobutanenitrile.
- Incipiently, dehydronation (deprotonation) takes place in 3-oxobutanenitrile by means of KOH. In a consequence of this change, the carbanion, a conjugate base, is generated; that plays its role as an active methylene in the compound.
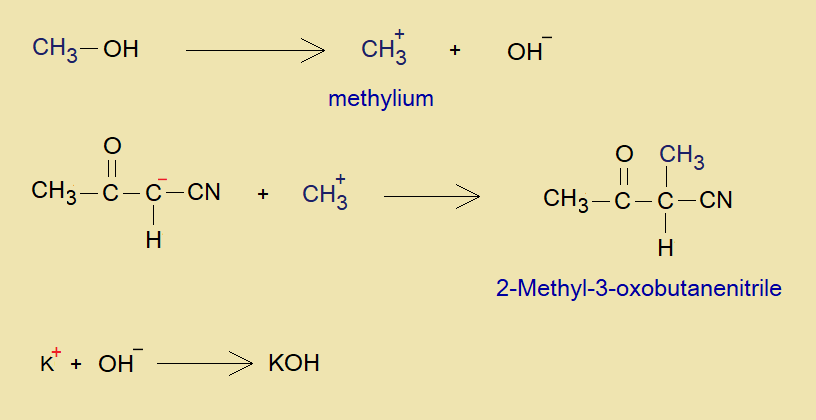
- The methanol is converted into methyl cation (methylium; carbanylium: CH3+). This electrophile makes a bond with nucleophile (carbanion) and ultimately the product is formed because of alkylation. This product is called 2-Methyl-3-oxobutanenitrile; KOH is recovered eventually by the reaction between K+ and OH– of methanol.
A Brief Account of Chemicals:
3-Oxobutanenitrile is a colourless to pale yellow liquid having boiling point 123.5°C at 1 atm. pressure. It is slightly soluble in water and is used in pharmaceutical industry. There are many other names of the compound, like, Cyanoacetone; 1-Cyano-2-propanone etc.
2-Methyl-3-oxobutanenitrile is a liquid having other names, 2-Cyano-3-butanone; 3-Cyano-2-butanone; 2-Methyl-3-keto-butyronitrile; or 1-Cyano-2-methyl-2-propanone. etc.



