Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Definitions of ionic bonds
- Cluster of ions
- Simple whole number ratio
- Sodium chloride formation
- Octet rule
- Dot & cross model
- Noble gases configurations & ions
- Isoelectronic particles
- Potassium oxide formation
- Magnesium fluoride formation
- Calcium oxide formation
- Chemical formula
Ionic Bond: Definition
- The oppositely charged ions have electrostatic forces of attractions to make a chemical bond called ionic bond.
- The cations and anions have electrostatic forces of attractions to make a chemical bond called ionic bond.
- The chemical bond that is formed by the complete transference of electron(s) from one atom to another to make oppositely charged ions that held together by electrostatic forces of attractions, is called ionic bond.
Cluster of Ions:
Ionic bond also known as “electrovalent bond”. The electrostatic attractions between oppositely charged ions make a cluster of cations and anions, as shown in the following diagram.

Exercise 1:
From the following three-dimensional structure, point out the mistakes created at two places.

Simple Whole Number Ratio:
Although, ionic bonds are cluster of ions but their formula is a simple whole number ratio. For example, NaCl, KCl, CaBr2, FeCl3, K2O etc.
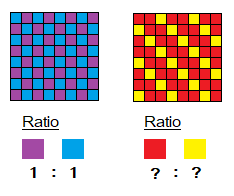
To understand the concept, look at tiles of the two walls. In 1st wall purple and blue tiles have ratio=1:1. Now, guess the ratio in second wall between red and yellow tiles. Likewise, the crystalline forms are the cluster of cations and anions and their simple whole number ratio is their formula.
There is in-detail description, why do atoms form ions and why it is essential; for this click on the link https://chemiologist.com/understanding-ions-and-their-formation/
1. Sodium Chloride Formation
The compound sodium chloride (NaCl) is a table salt and everyone is familiar. However, a chemist knows that it is a good example of ionic bond. Sodium is a metallic cation (Na+) while chloride is a non-metallic anion (Cl–); and thus, due to oppositely charged particles they attract each other to establish an ionic bond to form NaCl.
During a chemical reaction between sodium molten metal (Na) and chlorine gas (Cl2), the cations and anions are formed. Sodium loses one of its outermost shell electron and chlorine gains that electron. So, Na changes to Na+ while Cl changes to Cl–.
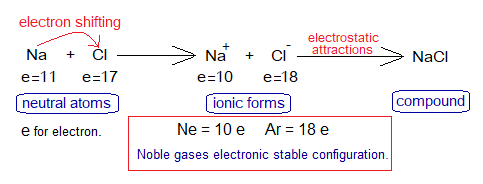
A neutral sodium atom has 11 electrons and loses 1 to attain stable electronic configuration of neon (Ne, electron = 10). Likewise, neutral chlorine atom has 17 electrons and needs 1 more electron to attain stable electronic configuration of argon (Ar, electrons = 18). This is the reason of electron transference.
Octet Rule:
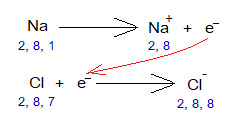
The outermost shell (OMS) should be filled by 8 electrons to complete shell or subshell. Go through the following change in electronic configurations of sodium and chlorine to come to know the 8 electrons in OMS of Na+ and Cl–. Also count 2+8=10 and 2+8+8=18 following the configurations of noble gases.
Dot & Cross Model:
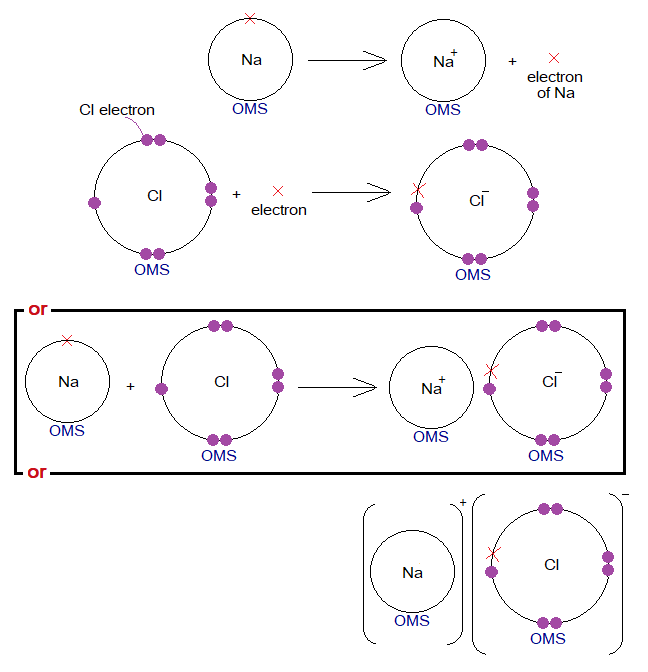
The electron transfer from metal atom(s) to non-metallic atom(s). The ionic bond formation can also be expressed by dot and cross model using OMSs of atoms. As, for NaCl the cross is used with Na for its electron; while dots are used for electrons of Cl; can be reversed as well.
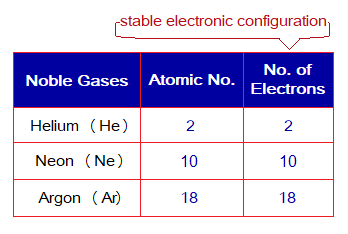
Noble Gases Configurations & Ions:
The noble gases are the only elements having their stable electronic configurations. So, all other elements follow these configurations (whichever is nearby) to make themselves stable, but by doing that they lose or gain electron(s) and make ions. Thus, during chemical change the oppositely charged ions make ionic bonds by electrostatic forces of attractions. The first three noble gases have the following electronic configurations.
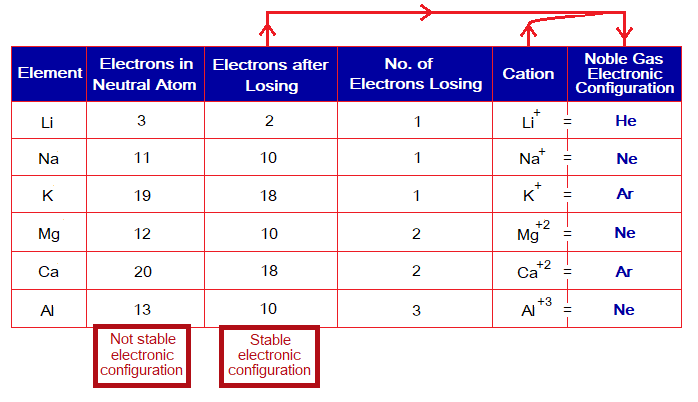
Metals lose electron(s) to attain stable configurations as shown in the following table:
Non-metals gain electron(s) to attain stable configurations as shown in the following table:
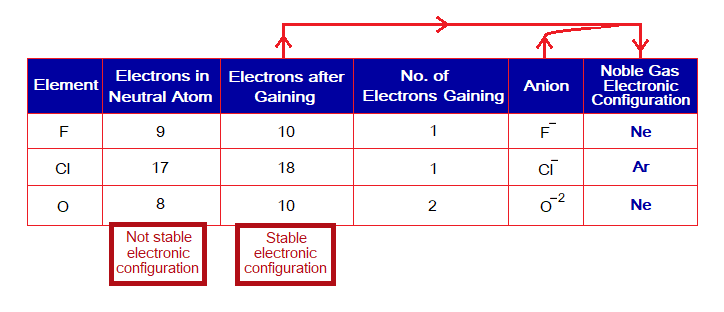
Isoelectronic Particles:
“The elements that attain noble electronic configuration and are similar with respect to their electronic configuration by this change are called isoelectronic particles”. For example, electronic configuration of Ne is by 10 electrons; and Na+, Mg2+, Al3+, F–, N3- (nitride ion of nitrogen), O2- are isoelectronic together by this concept; all have electrons 10. In another set, H–, Li+, Be2+ (beryllium) have their electronic configuration by 2 electrons and similar to He.
2. Potassium Oxide Formation
Potassium is a metallic cation (K+); while oxide is a non-metallic anion (O-2); and thus, due to oppositely charged particles they attract each other to establish an ionic bond to form K2O.
During a chemical reaction the cations and anions are formed. Potassium loses one of its outermost shell electron and oxygen gains two electrons from two K. So, K changes to K+ while O changes to O-2.
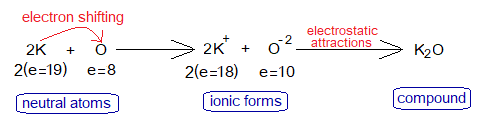
Oxygen needs two electrons to complete its octet. One potassium loses 1 electron; so, two potassium atoms lose 2 electrons. By this way K2O is formed. The change in electronic configuration is here as under:
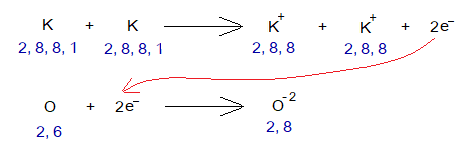
Exercise 2:
From the above chemical reactions, describe how the electronic configurations are satisfied among K and O?
The dot-cross diagram is shown below:

3. Magnesium Fluoride Formation
Magnesium is a metallic cation (Mg+2) while fluoride is a non-metallic anion (F–); and thus, due to oppositely charged particles they attract each other to establish an ionic bond to form MgF2. Fluoride is anion of fluorine atom.
During a chemical reaction the cations and anions are formed. Magnesium loses two of its outermost shell electrons and fluoride gains one electron from Mg. So, Mg changes to Mg+2 while F changes to F–.
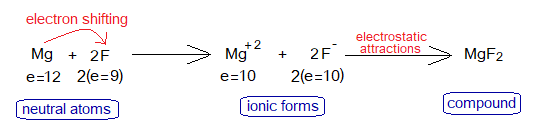
Magnesium needs two electrons to lose to complete its octet. Fluoride needs 1 electron to gain; so, both ions develop ionic relationship to form MgF2. Both ions, cation and anion are isoelectronic; look at the change in electronic configurations as under:
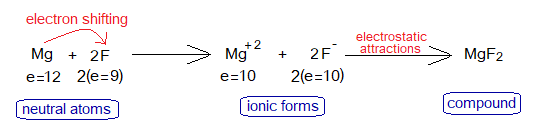
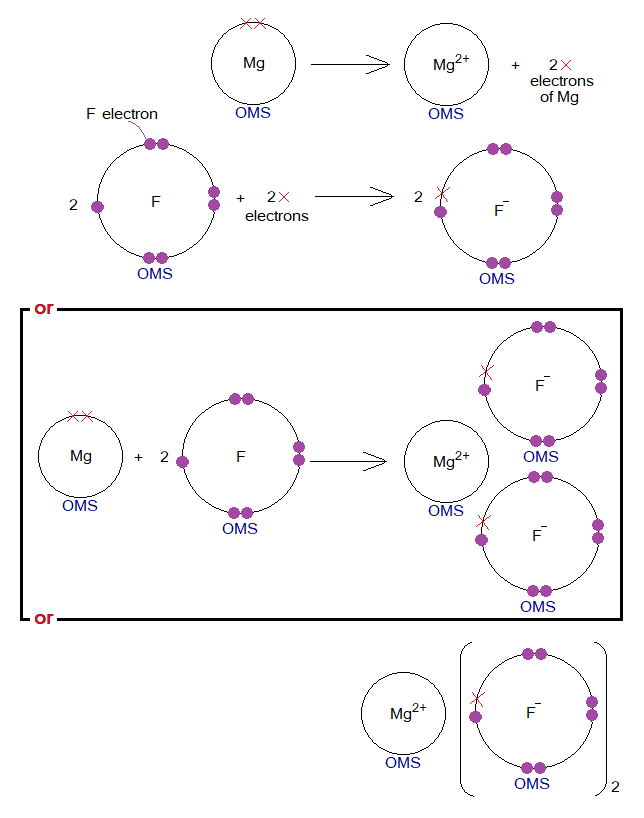
Exercise 3:
- From the above chemical reaction, describe how the electronic configurations are satisfied among Mg and F?
- Go through the following diagrams and draw the dot-cross diagram of MgF2 in brackets as in K2O.
The dot-cross diagram is shown below:
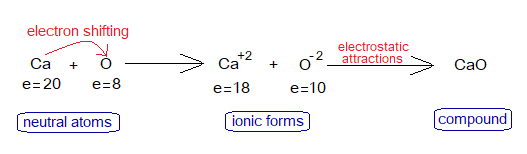
4. Calcium Oxide Formation
Exercise 4:
Explain the following flowsheet related to the formation of CaO an ionic compound.
Exercise 5:
Explain the following ionic chemical reactions in terms of changes in electronic configurations according to noble gases.

Exercise 6:
Draw the dot-cross diagrams of CaO as an ionic compound.
Exercise 7:
Explain ionic bond in aluminium oxide (Al2O3). Hint: It is an ionic bond between Al3+ and O2-.
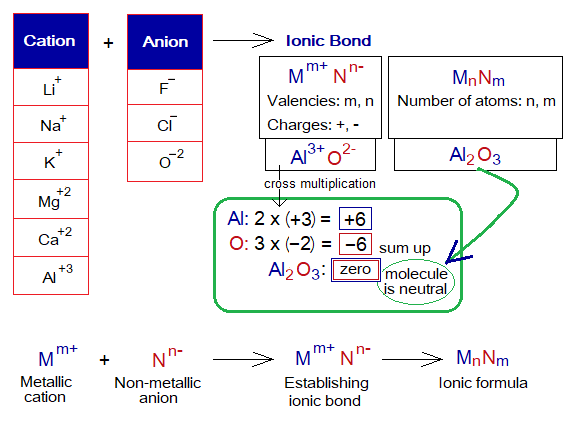
Chemical Formula:
As the electrovalent (ionic) bond is formed by electrostatic forces of attractions between cations and anions, but there is a need to know how the chemical formula is formed to make the ionic compound a neutral molecule.
- The valency is the combining capacity of the element, e.g., ‘m’ and ‘n’ for metallic cation and non-metallic anion respectively; might be 1, 2, 3 or more.
- The charges are positive and negative signs. Valency and charges together are called oxidation state or oxidation number.
- When chemical formula of ionic compound is written, the valency of anion becomes subscript of cation as its number (quantity) in the molecule. Likewise, the valency of cation becomes subscript of anion as its number. Remember, charges are not shifted. So, a formula is formed.
- The ionic compound formed is a neutral molecule, as explained in the following. How the ionic compounds are neutral; go through the following to understand the concept.
Exercise 8:
From various cations and anions using above list, form chemical formulas (also ‘formulae’ as plural) of various ionic compounds. Note: There is no need to write ‘1’ as valency or number for an atom; it is understood.



