Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Ions: Definition
- Oxidation state
- Types of Ions
- Cations: Definition
- Ionic chemical equation for cation formation
- Charge calculation for cation
- Types of cations
- Why mono, di, trivalent cations and anions are formed?
- Anions: Definition
- Ionic chemical equation for anion formation
- Charge calculation for anion
- Types of anions
What are Ions?
By definition,“when neutral atoms change their electronic configuration by gaining or losing electrons from their outermost shell, then ions are formed”. The outermost shell also called valence shell, last shell or highest energy shell.
Oxidation State: “The charge carried by an ion” is called oxidation state or oxidation number. For example, Ca2+, Cl–, 2+ and -1 are oxidation states of calcium and chlorine respectively.
Types of Ions:
There are two types of ions that are formed:
- Cations: positively charged ions.
- Anions: negatively charged ions.
- Cations: Definition: “When neutral atoms change their electronic configuration by losing electrons from their outermost shell, then cations are formed”. The phenomenon is called oxidation.
The cation formation can be expressed in the form of ionic chemical equation, as under:
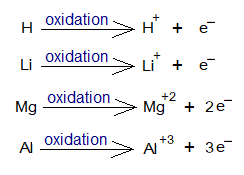
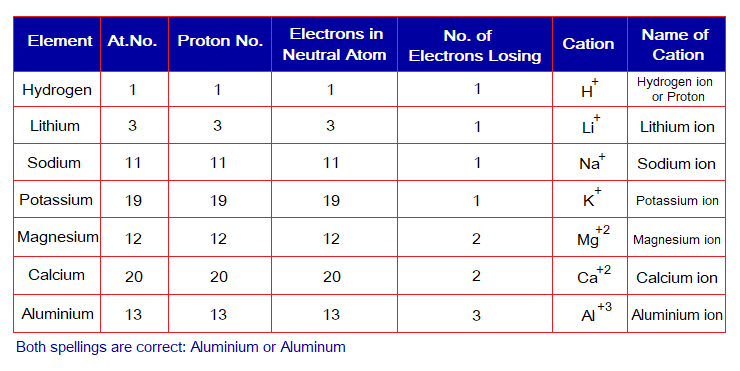
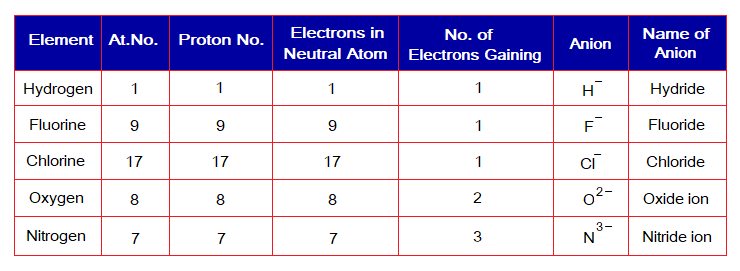
The following table describes the phenomenon. Note: The H+ doesn’t carry electron and consisted of only a single proton; thus, hydrogen ion also known as ‘proton’.
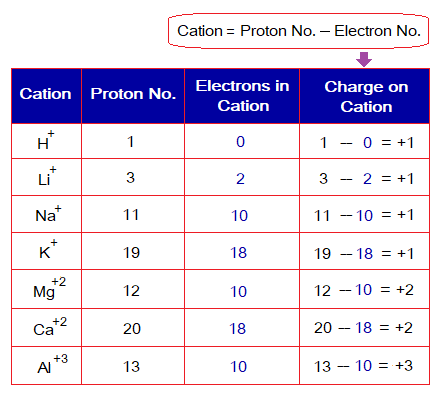
Charge Calculation for cation: When the number of electrons is less than the number of protons, cations are formed by oxidation. In other words, the cation carries fewer electrons than protons. According to the procedure, the electron number (count) is subtracted from the proton number to know the charge obtained, as described in the following table.
Following are the types of cations:
- Monovalent Cations: The cations having oxidation number +1. For example, H+, Li+, Na+, K+, Ag+ (silver) etc. There is no need to write 1, it is known.
- Divalent Cations: The cations having oxidation state +2. For example, Mg2+, Ca2+, Zn2+ (zinc) etc. These can also be written in a different style like Mg+2, Ca+2, Zn+2, but not preferable, an old style; the same is true for anions.
- Trivalent Cations: The cations having oxidation number +3. For example, Al3+, Cr3+, Co3+, Bi3+ etc., chromium, cobalt, bismuth cations respectively.
Why Mono, Di, Trivalent etc. Cations and Anions are formed?
All the atoms don’t have their stable electronic configuration except Noble gases. 2, 10, 18, 36 …… are stable belonging to He, Ne, Ar, Kr etc. respectively. So, all the atoms try to attain the nearby stable noble gas electronic configuration. That’s why cations and anions are formed. Few examples are given in the following table, try to go through the change taking place by losing electrons and attaining a stable noble gas configuration. Hydrogen loses its single electron to gain +1 charge; because, 1 electron in K-shell is not following noble gas configuration. However, H– (hydride ion) can also be formed and it will be discussed in anion section.


- Anions: Definition: “When neutral atoms change their electronic configuration by gaining of electrons to their outermost shell, then anions are formed”. The phenomenon is called reduction.
The anion formation can be expressed in the form of ionic chemical equation, as under:
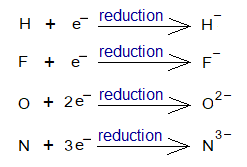
The following table describes the phenomenon.
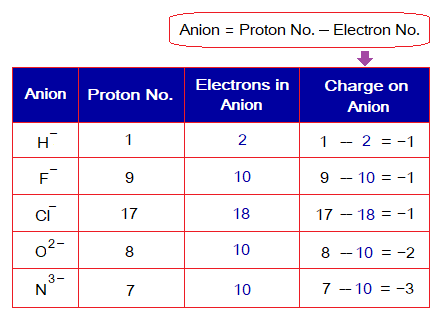
Charge Calculation for Anion: When the number of electrons is greater than the number of protons, anions are formed. In other words, the anion carries fewer protons than electrons. According to the procedure, the electron number (count) is subtracted from the proton number to know the charge obtained, as described in the following table.
Following are the types of anions:
- Monovalent Anions: The anions having oxidation number -1. For example, H–, F–, Cl– etc.
- Divalent Anions: The anions having oxidation state -2. For example, O2- etc.
- Trivalent Anions: The anions having oxidation state -3. For example, N3- etc.
Exercise 1:
Describe the stable electronic configurations by noble gases in the following ions:
- anions: hydride, fluoride, chloride, oxide, and nitride;
- cations: potassium, sodium, and magnesium.