Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Atomic mass
- Atoms Mass Unit (amu)
- Calculating number of neutrons
- Symbolization
- Mendeleev periodic table
Atomic Mass
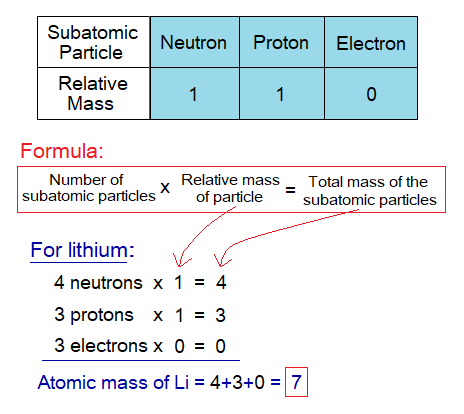
- By a simple definition, “the sum of the number of protons and neutrons of an atom of an element is called atomic mass”. The atomic mass is written by a symbol ‘A’; and aka Mass Number or Nucleon Number; i.e., the total number of subatomic particles in the nucleus. For example, the atomic mass of Lithium is 7; because, it has 4 neutrons and 3 protons. The atomic mass of carbon is 12 by sum up its 6 neutrons and 6 protons. The calculations of atomic masses of first twenty elements are given in the following tables. Remember, Hydrogen is the only known atom having zero neutron in its protium isotope.
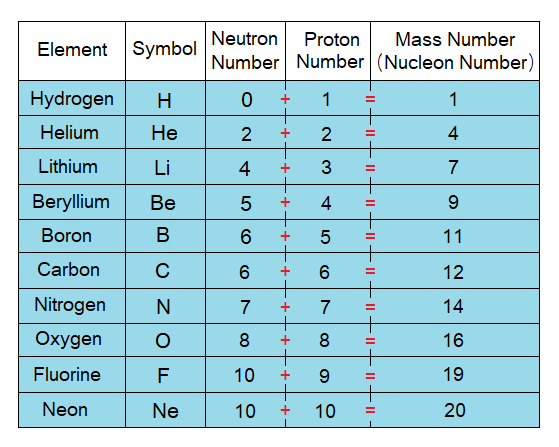
By a more comprehensive definition, “the atomic mass is the sum of relative masses of all the subatomic particles in an atom”. It will give the same calculation results as the above one. For example, the lithium has 4 neutrons, 3 protons and 3 electrons. The relative mass of neutron is 1, the relative mass of proton is also 1, while the relative mass of electron is zero. So, it will calculate 7 the atomic mass by using a formula as under and then by adding obtained masses of all the subatomic particles together to get a total:
Exercise 1:
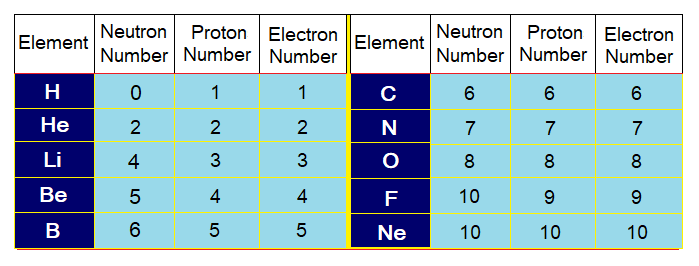
By using the following table and above formula, calculate the atomic masses of first 10 elements similar on the pattern of Li.
Exercise 2:
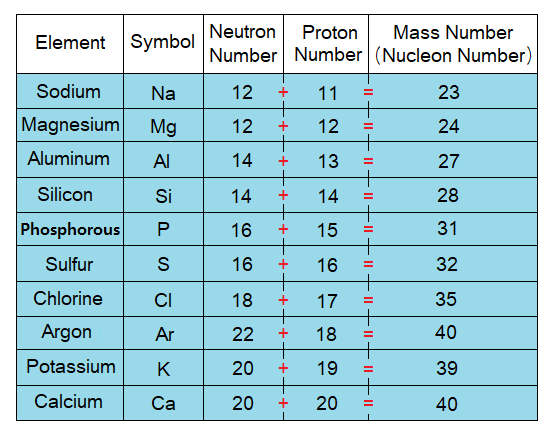
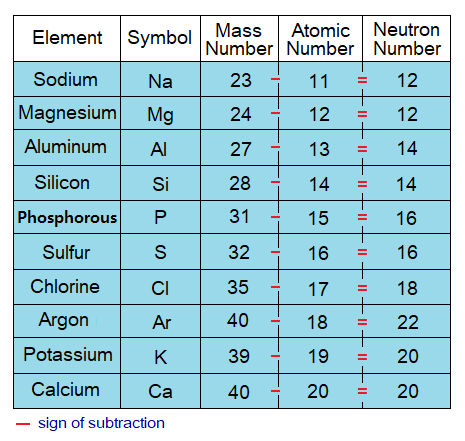
The elements having atomic numbers 11 to 20 are sodium to calcium. Write the elements’ symbols, number of protons, electrons, and neutrons. Tally your answer with the information given on the link https:/atomic-number-and-its-use-in-periodic-table/. Hint: (1) The number of protons is the atomic number; further, (2) the number of protons and electrons are same in a neutral atom. The procedure how to find out mathematically the number of neutrons is given below.
Atomic Mass Unit:
The atomic mass is expressed in atomic mass unit (amu), e.g., Li = 7amu, Na = 23amu.
Calculating Number of Neutrons:
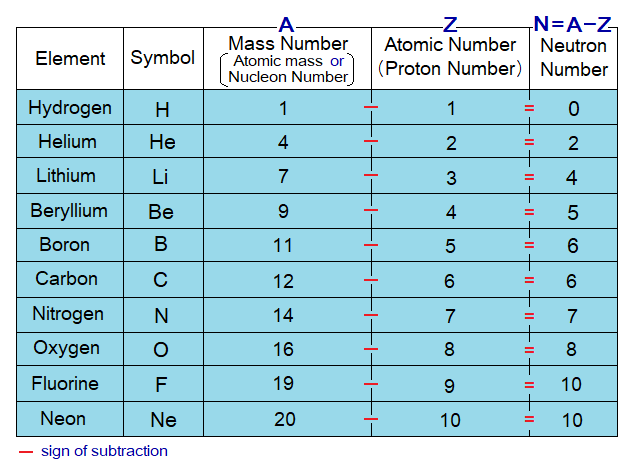
If atomic number (Z) is subtracted from atomic mass (A) of an atom, then its number of neutrons (N) will be obtained. Because, atomic number is actually a proton number; and atomic mass is a total of nucleon particles, i.e., sum of neutrons and protons. Thus, subtraction provides the count of neutrons in that atom. Pervade thoroughly the following tables to understand the concept of neutron number calculation for the first 20 elements; i.e., to know how many neutrons exist in an atom of a particular element.
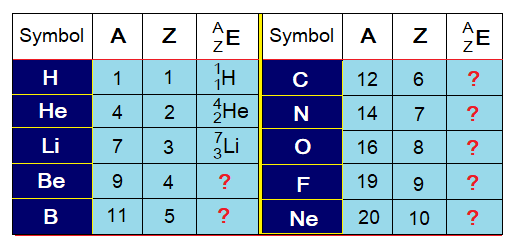
Symbolization:
Z is the symbol of atomic number, A is the symbol of atomic mass; and if E might be used as the symbol of an element, then, in chemistry, it is expressed as in the following table. By the rule, the A and Z both are written on the left side of the element’s symbol. However, A is shown on the upper side while Z on the lower side.
Exercise 3:
Go through the concept of writing symbolic form of an element with its atomic mass and atomic number from the following table; and solve the question marks.
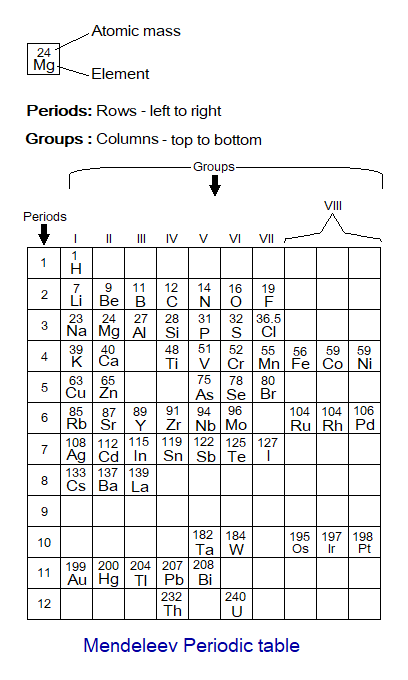
Mendeleev Periodic Table:
A Russian Chemist, Dmitri Ivanovich Mendeleev (1834-1907) arranged elements according to similarities in their properties and developed a periodic table based upon increasing in their atomic masses. He defined the Periodic Law by means of atomic masses and placed elements in groups and periods based upon resemblance in the properties. The vertical columns (top to bottom) were called groups; while the horizontal rows (left to right) were named periods. He kept few blank spaces for undiscovered elements for future placement when will be discovered. Later on, an English Physicist, Henry Gwyn Jeffreys Moseley (1887-1915) changed the theme and arranged elements according to increase in their atomic numbers and formulate Modern Periodic Law, still in function. Open up the link to view Moseley’s periodic table https:/atomic-number-and-its-use-in-periodic-table/.