Synthesis of ß-Hydroxyester
Dr. Mudassar Altaf, Associate Professor, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Definition and overall reaction
- Mechanism
Definition and Overall Reaction:
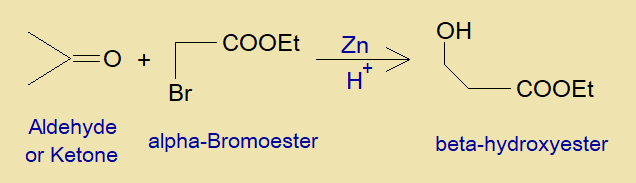
Reformatsky Condensation Reaction was developed by a Russian chemist Sergey Nikolaevich Reformatskii (1860-1934). It is a condensation between aldehyde or ketone and alpha-bromoester to produce beta-hydroxyester in acidic media in the presence of zinc.
Mechanism:
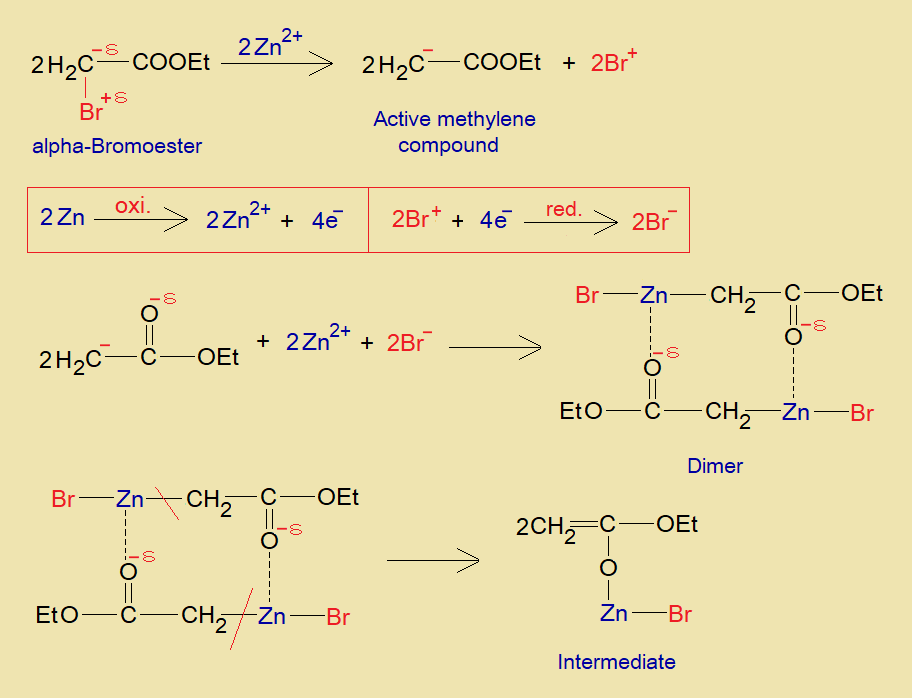
- The α-bromoester changes to active methylene by eliminating bromonium ion (Br+) in the presence of zinc metal.
- Zn is oxidized by 2 electrons that further reduce two moles of bromonium ions into bromide ions.
- Zinc cation makes a bond with carbanion and one bromide ion just like magnesium forms Grignard reagents. Further, due to electropositive character of the metal, to the second molecule, an intermolecular attraction is established between partial negative oxygen of the ester group and the zinc. A dimer is formed by two molecules as shown in the mechanism.
- Thereafter, zinc breaks its bond with alpha-carbon of ester from both sides of the dimer and establishes its bond with oxygen by making unsaturation in alpha-carbon and ester group carbon. This is an intermediate molecule.
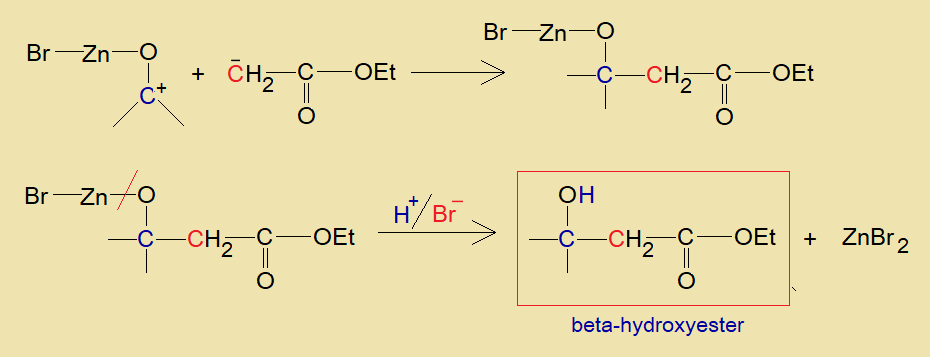
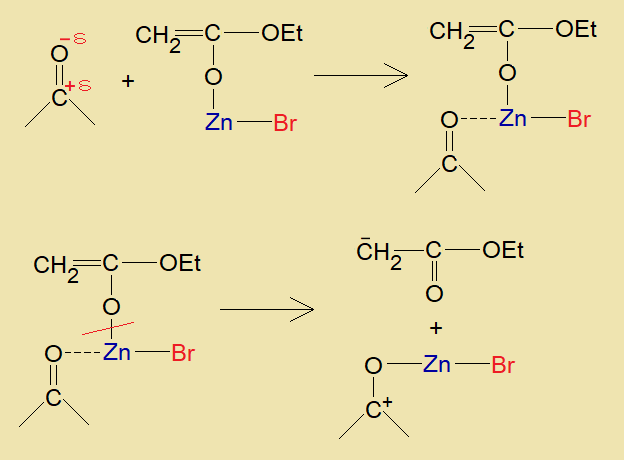
- Now, zinc of this intermediate organic compound establishes intermolecular attractive forces with partial negative oxygen of another aldehyde or ketone.
- Resultantly, zinc breaks its bond with ester molecule and makes its bond with aldehyde or ketone. The aldehyde or ketone carbonyl-carbon carries positive charge on it by this bond formation; and the ester is formed carrying a negative charge on its alpha-carbon.
- Both the opposite charges of the carbons make a carbon-carbon link; oxygen-zinc bond breaks and in the presence of proton of an acid the beta-hydroxyester is formed. Zinc forms zinc bromide, as a bromide ion was existing there.