Dr. Mudassar Altaf, Associate Professor, Department of Higher Education, Government of the Punjab, Pakistan
The copy of the content is not allowed
Contents:
- Introduction
- Synthesis
- Mechanism
Introduction:
Cinnamic acid is an organic compound exists naturally in cinnamon (dar-cheeni in Urdu, Hindi). In lab, this compound was synthesized by an English chemist William Henry Perkin (1838-1907). Cinnamic acid has its chemical formula C9H8O2. The crystalline structure has its white powdered appearance with m.pt. 133°C & b.pt. 300°C; the solubility in water is 50mg/100ml. By its structural formula, it is a benzene ring having acrylic acid; so, it is 3-Phenylacrylic acid.
Synthesis:
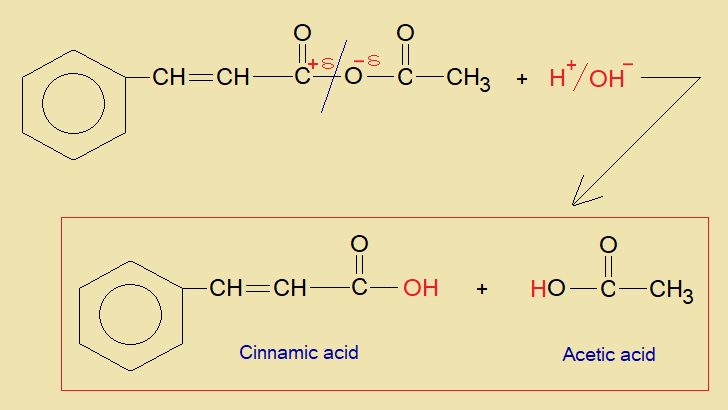
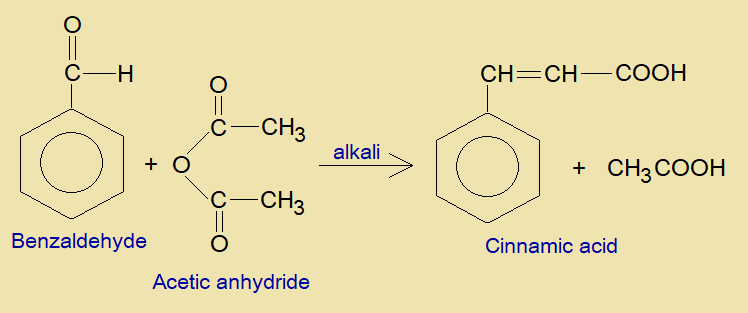
In the presence of a base, benzaldehyde and acetic anhydride are condensed together to form cinnamic acid along with acetic acid.
Mechanism:
- Acetic anhydride undergoes into deprotonation from one side by a base. Resultantly, α-carbon changes to carbanion that will act as active methylene.
- Carbanion attacks on partial positive carbon of carbonyl of benzaldehyde and ultimately makes an unsaturated intermediate compound by the elimination of hydroxide ion.
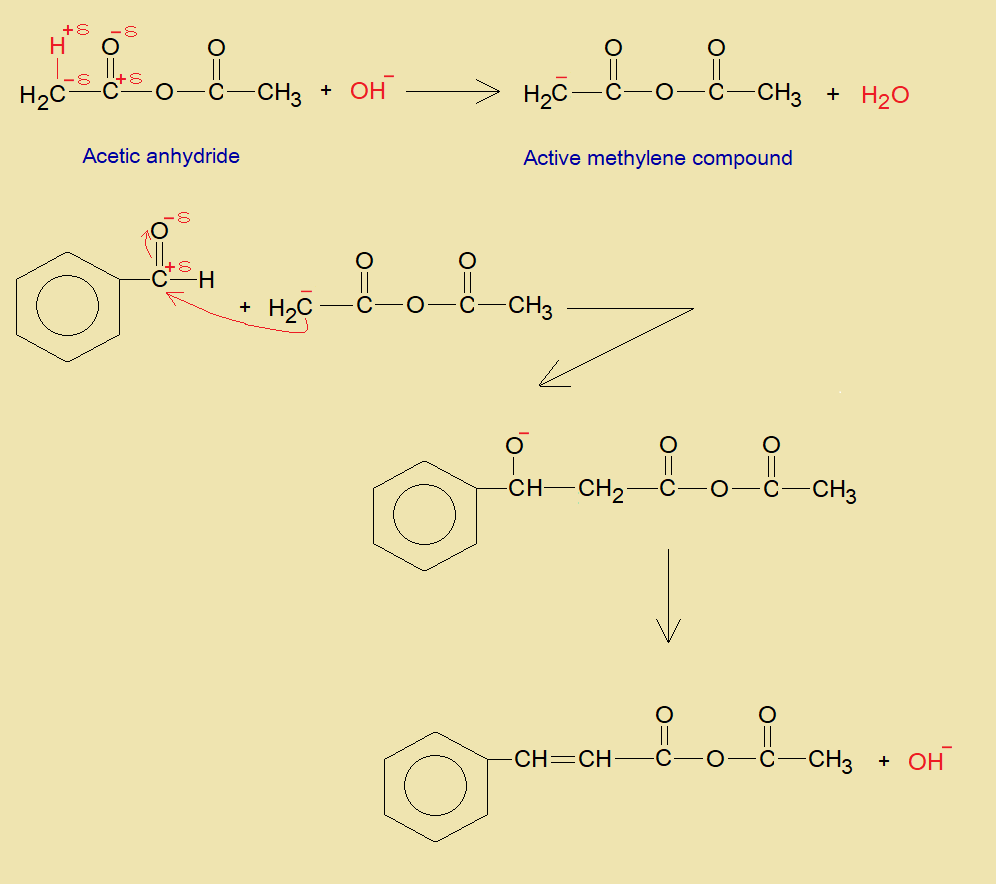
- By hydration of the intermediate compound, the acetic acid and cinnamic acid are formed.