Dr. Mudassar Altaf, Associate Professor of Chemistry
The copy of the content is not allowed
Paper: P3 Oct./Nov. 2022 9701/36
Question statement: In the experiment, you will determine the concentration of dilute HCl by further diluting it and then titrating with aqueous K2CO3.
Solutions provided:
- FB 1: dilute HCl solution.
- FB 2: 8.46g dm-3 anhydrous K2CO3 solution.
- FB 3: Bromophenol blue indicator.
(a) Method:
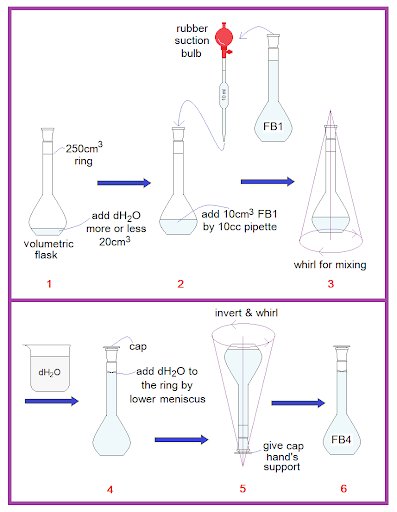
- Use 10cm3 pipette to transfer 10.0cm3 of FB 1 into volumetric flask.
- Make the solution up to 250 cm3 using distilled water.
Shake the volumetric flask and its contents thoroughly. Label this solution FB 4.
- Fill the burette with FB 4.
- Pipette 25.0 cm3 of FB 2 into conical flask.
- Add few drops of FB 3.
- Perform rough titration and record your burette readings in the space below
- The rough titre is ………………….. cm3.
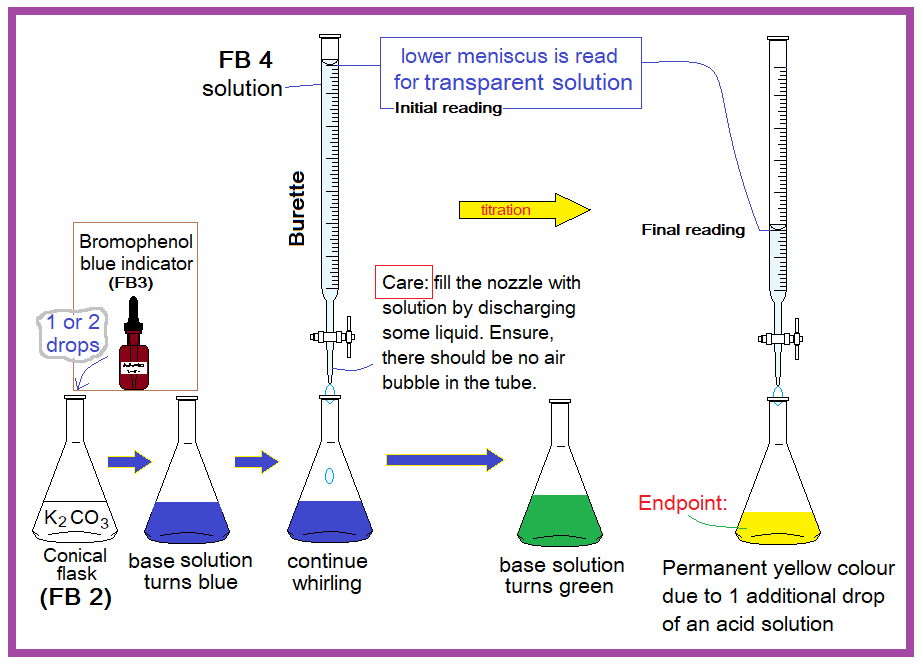
- Carry out as many accurate titrations as you think, necessary to obtain consistent results.
- Make sure any recorded results show the precision of your practical work.
- Record in a suitable form below all your burette readings and the volume of FB 4 added in each accurate titration.
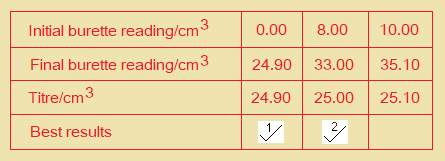
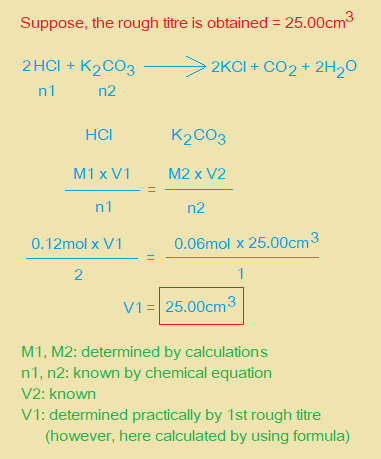
(b) From your accurate titration results, calculate a suitable mean value to be used in your calculations. Show clearly how you obtained this value.

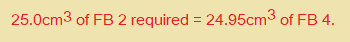
25.0cm3 of FB 2 required ………………………. cm3 of FB 4.
(c) Calculations:
i. Give your answers to c(ii), c(iv) & c(v) in appropriate number of figures.
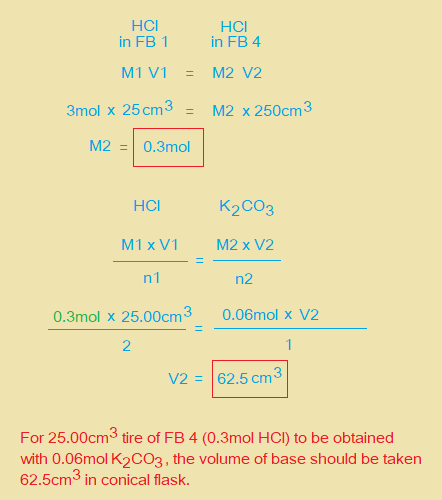
ii. Use information on page 2 to calculate the amount in mol of K2CO3 present in 25.0cm3 of FB 2. Amount of K2CO3 = ……………. mol.
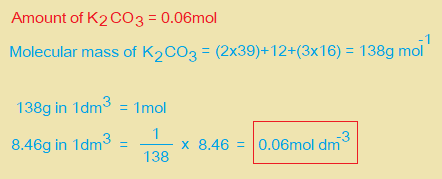
iii. Give the ionic equation for the reaction taking place in the titration in (a). Include state symbols.
………CO32-……..+………..H+………..→…………+…………
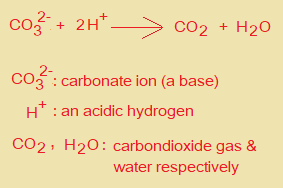
iv. Calculate the concentration in mol dm-3 of HCl in FB 4.
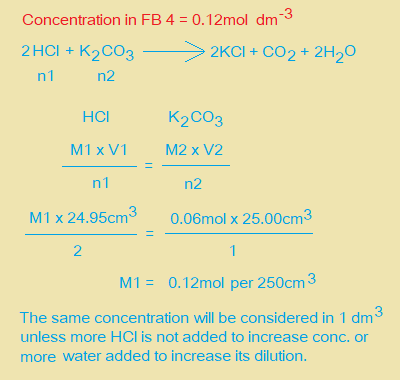
v. Calculate the concentration in mol dm-3 of HCl in FB 1.
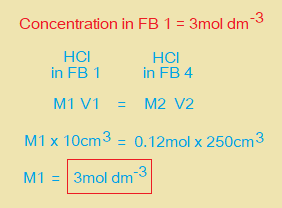
(d) A student uses a solution containing 8.46g dm-3 of hydrated K2CO3 solution in the titration of (a) instead of FB 2. State, whether the student’s titre would be larger or smaller than your titre. Explain your answer.
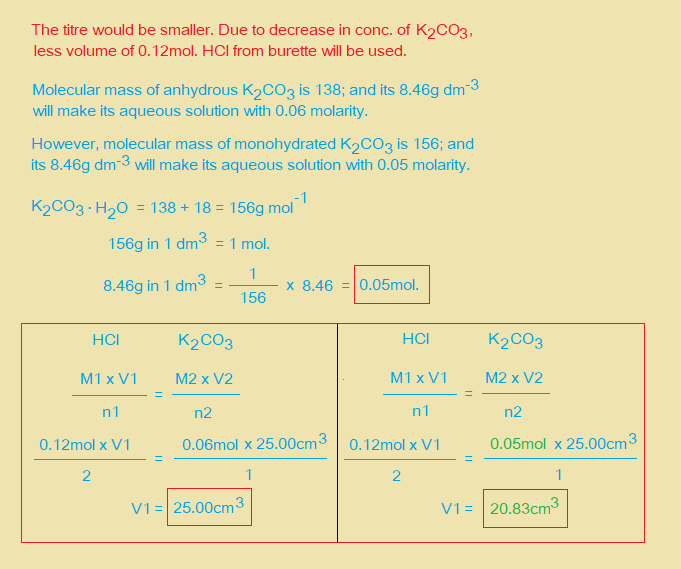
(e) A student carries out the titration described in (a). By mistake, a 25cm3 pipette is used to transfer 25.0cm3 (instead of 10.0cm3) of FB 1 into volumetric flask. The solution is made up to 250cm3 with distilled water and labelled as FB 4. State, how the subsequent experimental procedure could be modified so that the titre obtained in (b) is not altered by the mistake.