Dr. Mudassar Altaf, Associate Professor of Chemistry, Department of Higher Education, Government of the Punjab, Pakistan
Contents:
- Electrolysis: definition
- Apparatus for electrolysis
- Electrolytes: definition
- Types of electrolytes
- Redox phenomenon
- Ionic half reactions
- Electrolysis of molten lead bromide
- Electrolysis of NaCl aqueous solution
- Electrolysis of dilute H2SO4 solution (electrolysis of water)
- Electrolysis of CuSO4 solution using graphite electrodes
- Electrolysis of CuSO4 solution using copper electrodes
- Application of using Cu anode
- Electroplating: (electrodeposition or electrochemical deposition), definition
- Procedure of electroplating
- Chromium electroplating (chrome plating), nickel electroplating
Electrolysis:
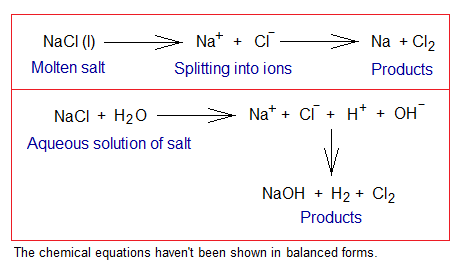
Definition: It can be defined as, “the chemical decomposition of ionic compounds by passing an electric current from their liquid state or aqueous solution to form new products”. For example:
- If electric current is passed through molten NaCl salt, then the compound first splits into its ions and thereafter chemical changes occur; consequently, sodium metal and chlorine gas are formed.
- If electric current is passed through aqueous solution of NaCl salt, then the compound and the water molecules first split into ions and thereafter chemical changes occur; consequently, sodium hydroxide (NaOH), hydrogen and chlorine gases are formed.
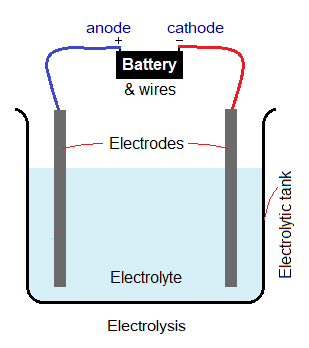
Apparatus for Electrolysis:
In a more general form, the apparatus for electrolysis is consisted of the following component parts:
- Electrolytic Cell: It is a cell that is filled with electrolyte for the process of electrolysis.
- Electrodes: There are two electrodes, anode and cathode, dipped into the electrolyte; former is positive; while latter is a negative electrode. The electrodes are connected with battery by means of wire outside the cell. These are inert materials and don’t take part in chemical reactions during the electrolysis. Mostly, platinum metal or graphite (allotropic form of carbon) electrodes are used.
- Battery: Mostly, a 12 volts DC power supply is used.
- Wires: Wires are connected with terminals of the battery and to the electrodes.
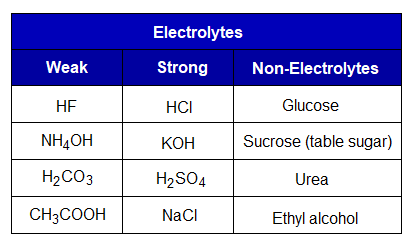
Electrolytes: These can be defined as, “the substances, in either molten or aqueous solution state, allow electric current to pass through them and split into ions to bring about chemical changes to form new products”.
Types of Electrolytes:
The electrolytes are either weak or strong depending upon their strength to split into ions. More the splitting, to that extent the electrolyte is strong; and vice versa.
- Weak Electrolyte: “A substance that dissociates into ions partially during electrolysis”.
- Strong Electrolyte: “A substance that dissociates into ions completely during electrolysis”.
- Non-Electrolyte: “A substance that doesn’t dissociate into ions during electrolysis”.
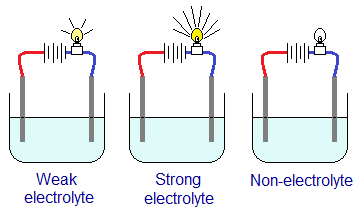
Whenever, a bulb is connected with the circuit, its brightness indicates the level of ionization of an ionic electrolyte during electrolysis. The bulb lights up bright with strong electrolyte; dim with weak; and doesn’t light up with non-electrolyte.
Redox Phenomenon:
The electrolysis is a redox phenomenon. Redox is a short form of reduction & oxidation by the combination of red+ox. In terms of electron(s) transfer, these can be defined as:
- Oxidation: The loss of electron(s) from an atom, ion, or molecule. It occurs at anode. In redox, it is ionic half reaction. Mostly, non-metallic anions show oxidation. However, when copper anode is used during electrolysis of CuSO4 aqueous solution, the anode metal itself undergoes into oxidation.
- Reduction: The gain of electron(s) by an atom, ion, or molecule. It occurs at cathode. In redox, it is another ionic half reaction. Hydrogen ion (H+) and metallic cations show reduction.
In the following example of lead(II) bromide electrolysis, lead cation [Pb2+ or Pb(II)] having metallic nature shows reduction by 2 electrons to change into neutral lead (Pb). These two electrons come by the oxidation of non-metallic bromide ions to change into neutral bromine atoms. Following that the bromine atoms form bromine molecule (Br2) by covalent bonding.
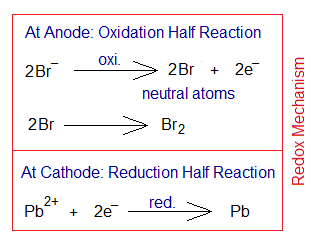
Electrolysis of Molten Lead Bromide:
This is white crystalline inorganic solid; having melting point is 370.6°C. When its molten liquid is electrolyzed, the overall reaction shows that bromine gas and lead are produced.
Further, the following step-by-step procedure takes place:
- Ionization: One mole of PbBr2 dissociates into one mole Pb2+ (lead cation) and two moles of Br– (bromide ions).
- Oxidation: The anions (bromide ions) travel towards positive electrode (anode). There, at anode, the oxidation takes place by 1 electron per bromide ion. So, two moles of neutral bromine atoms are formed; which ultimately form a covalent bond to generate 1 mole of bromine gas (Br2).
- Reduction: The lead cations travel towards negative electrode (cathode). There, at cathode, the reduction takes place by 2 electrons per Pb2+. Subsequently, one mole of neutral lead atoms is formed.
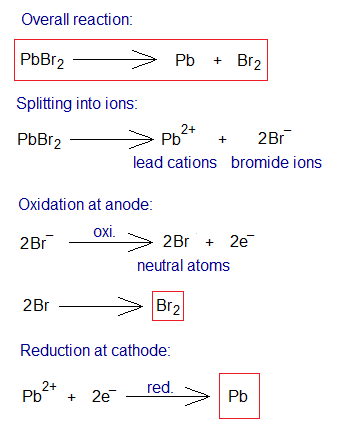
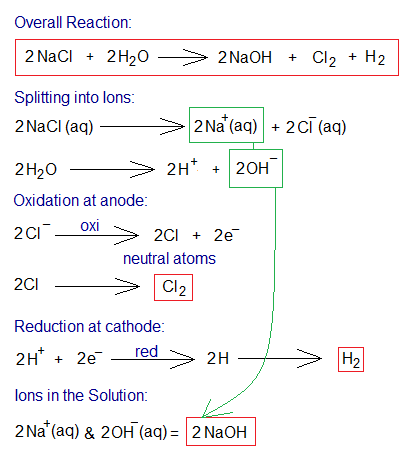
Electrolysis of NaCl Aqueous Solution:
An inorganic compound, sodium chloride, also called table salt, is a white crystalline solid in its pure form; and having its solubility in water 36g/100cm3 at 25°C (6.16 molar solution). For the electrolysis, 4-5 molar solution is used. The electrolysis yields two gases, chlorine and hydrogen; and the solution also contains ions Na+ and OH– (in other words, sodium hydroxide). The solution of table salt in water is called brine; i.e., saltwater. Both, brine and saline are solutions of table salt; but brine is more concentrated to saline. Saline is more purified, sterile and used in medical field. Further, during electrolysis, the following step-by-step procedure takes place:
- Ionization: (a) Two moles of NaCl dissociate into ions, two moles Na+ (sodium cations) and two moles of Cl– (chloride ions). (b) Two moles water dissociate into two moles of hydrogen ions (H+) and two moles of hydroxide ions (OH–).
- Oxidation: The anions (chloride ions) travel towards positive electrode (anode). There, at anode, the oxidation takes place by 1 electron per chloride ion. So, two moles of neutral chlorine atoms are formed; which ultimately form a covalent bond to generate 1 mole of chlorine gas (Cl2).
- Reduction: The H+ ions travel towards negative electrode (cathode). There, at cathode, the reduction takes place by 1 electron per hydrogen ion. Subsequently, two moles neutral hydrogen atoms are formed; which ultimately form 1 mole of hydrogen gas by covalent bonding.
- Ions in the Solution: The solution contains, at the end, Na+ and OH– ions (i.e., two moles of NaOH).
A simple lab glassware apparatus containing electrodes of carbon can be used, as shown below.
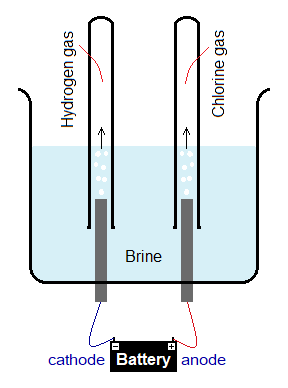
The electrolysis of brine solution can be conducted in Nelson cell; as well as in Hooker cell. Hooker cell was designed by an American Hooker chemical company in early 20th century for the production of NaOH (caustic soda). Both are the types of diaphragm cells.
Electrolysis of Dilute H2SO4 Solution:
The electrolysis of dilute sulphuric acid is actually the electrolysis of water. The pure water doesn’t undergo into ionization unless containing some other substance making its dissociation, like H2SO4. Protonation of water: the acid donates proton(s) to water to form hydronium ion (aka hydroxonium ion, simplest oxonium ion); which then starts further ionization into H+ and OH– ions. The final products of water electrolysis are hydrogen and oxygen gases.
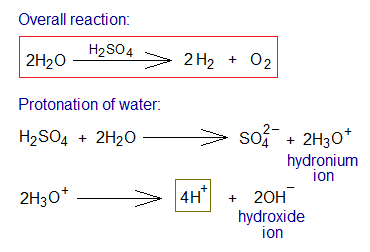
Further step-by-step procedure is given below:
- Ionization: Two moles of H3O+ ions dissociate into 4 moles of H+ and 2 moles of OH– ions.
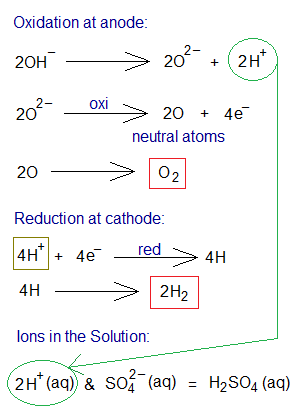
- Oxidation: Two moles of hydroxide ions travel towards anode; and dissociates into 2 moles oxide ions (O2-) and 2 moles protons (H+). There, at anode, the oxidation takes place by 2 electrons per oxide ion. So, 2 moles of neutral oxygen atoms undergo into covalent sharing and form 1 mole of oxygen gas (O2).
- Reduction: Four moles of H+ ions (were generated from 2 moles of H3O+ ions) travel towards cathode. There, at cathode, the reduction takes place by 1 electron per hydrogen ion. Subsequently, 4 moles neutral hydrogen atoms are formed; which ultimately form 2 moles of hydrogen gas by covalent sharing.
- Ions in the Solution: The solution contains, at the end, 2 moles of H+ and 1 mole SO42- ions (i.e., 1 mole of H2SO4 regenerated).
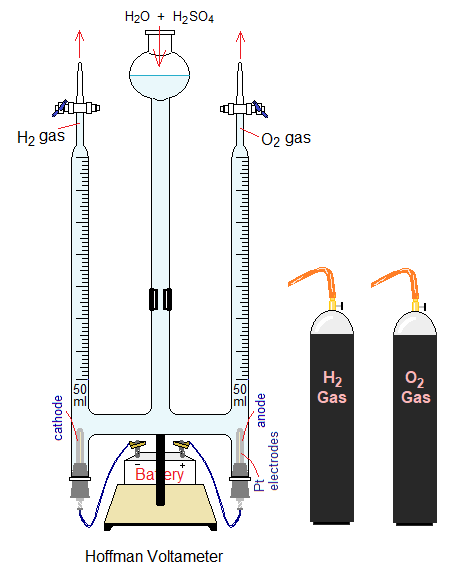
This electrolysis is conducted in Hofmann voltameter (also written as Hoffman) with platinum electrodes, designed in 1866 by a German chemist August Wilhelm von Hofmann (1818 – 1892), shown below in the diagram; a three glass tubes apparatus. Voltameter should not be confused with voltmeter; former is a glassware apparatus used for electrolysis of water, while latter is a device used to measure volts (voltage, electric potential) of electric current in the circuit.
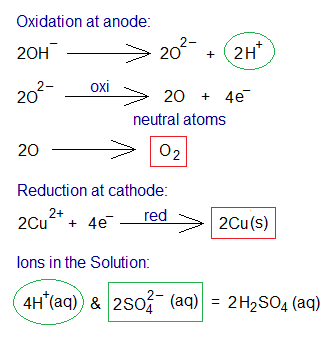
Electrolysis of CuSO4 Solution Using Graphite Electrodes:
The electrolysis of copper sulphate aqueous solution using carbon (graphite) electrodes yields pure copper metal and oxygen gas as products; whilst in solution the hydrogen and sulphate ions exist. Copper sulphate and water dissociate into their ions in the solution as shown below.
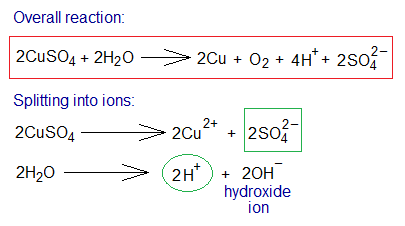
Step-by-step procedure of the chemical changes are given as under:
- Oxidation: The hydroxide ions (2 moles) move towards anode and dissociate further into oxide and hydrogen ions. The oxide ions show oxidation by 2 electrons per ion. Ultimately, these neutral oxygen atoms link up covalently to form oxygen gas molecules. By 2 moles of CuSO4; 1 mole of O2 is produced.
- Reduction: The copper cations move towards cathode and show reduction by 2 electrons per ion. So, neutral copper atoms (Cu metal) are formed.
- Ions in the Solution: The solution carries hydrogen as well as sulphate ions by 4 and 2 moles respectively.
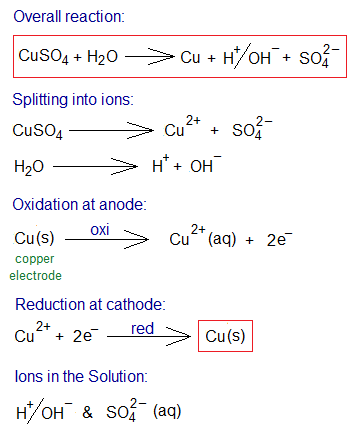
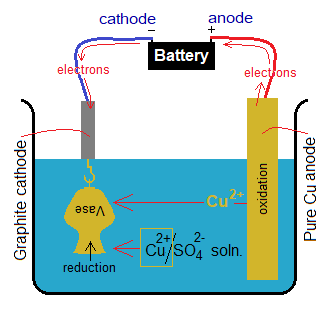
Electrolysis of CuSO4 Solution Using Copper Electrodes:
The electrolysis of copper sulphate aqueous solution using copper electrodes yields pure copper metal only as a product; whilst the oxygen gas is not produced. However, the solution carries the same ions, hydrogen and sulphate. Copper sulphate and water dissociate into their ions in the solution as shown below. Further, step-by-step chemical changes that occur during electrolysis are given below:
- Oxidation: The copper anode itself shows oxidation and releases copper cations into the solution. The electrons released, travel through external wire and re-enter into the solution via cathode for reduction of Cu2+ ions.
- Reduction: The Cu2+ ions move towards cathode. There are two sources of Cu2+ ions production in the solution: one by dissociation of CuSO
4,and second by oxidation of copper anode. So, the reduction of Cu2+ ions takes place to generate copper metal atoms; and pure copper metal is obtained resultantly. The pure copper, produced, deposits at cathode; and consequently, the thickness of cathode increases.
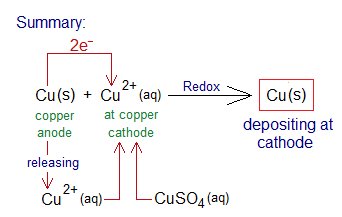
The overall mechanism can be summarized by the following:
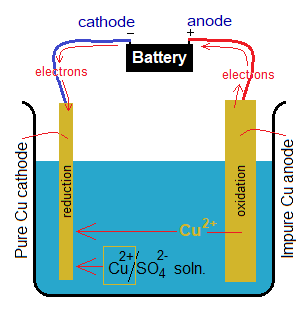
Application of Using Cu Anode:
The use of copper anode during electrolysis of copper sulphate solution has its industrial application of purifying copper. The anode is made by impure copper; while cathode is made by pure copper metal. As a result of electrolysis, the oxidation takes place at impure copper anode and consequently, the copper cations are produced, which are released in the solution and move towards cathode for reduction. Thus, pure copper, produced, going to be deposited on the pure cathode and increasing its thickness resultantly. The Cu2+ ions of electrolyte also move towards cathode for reduction. All the reactions are given above.
Electroplating:
By definition, “it is a process of depositing a thin layer of metal onto the surface of another metal by using electrolysis”. It is also called electrodeposition or electrochemical deposition process.
The procedure is simple as under:
- The aqueous solution of an electrolyte is filled in the electrolytic cell which would be used to deposit a thin layer by its metallic cation; for example, Cu2+ of CuSO4.
- The anode is made of the same metal as the one present as the electrolyte, for example Cu-anode in this case.
- The cathode is the object on which the thin layer of another metal has to be deposited. For example, to deposit copper on that particular cathodic-object.
In the following diagram, an iron vase is under electroplating of copper. Go through the details of chemical changes, particularly oxidation-reduction ionic half reactions involved.
In chromium electroplating (aka chrome plating), the thin layer of chromium is electroplated over objects of other metals, like brass, iron, nickel etc. Its purpose is to prevent the objects from corrosion; gives decorative appearance due to shiny look. The chrome plating has its wide application on automobile parts, kitchen utensils, toilet accessories like faucets, weapons, working tools either industrial or domestic etc. The same principle and purposes are true for nickel electroplating,where a thin layer of nickel is electrodeposited on the surfaces of other metallic objects.



